Human immunodeficiency virus, hepatitis B virus, hepatitis C virus rapid joint detection kit and its preparation and application
A technology of immunodeficiency virus and hepatitis B virus, which is applied in the field of biotechnology detection, can solve the problems of being unsuitable for clinical testing and large-scale blood screening, cumbersome operation, and high total cost, so as to reduce the risk of cross-contamination and detect High sensitivity and the effect of reducing manual errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 kit
[0048] Synthesize HIV detection primers and probes, HBV detection primers and probes, HCV detection primers and probes respectively, and their nucleotide sequences are shown in Table 1 below:
[0049] Table 1
[0050]
[0051] The above-mentioned sets of primer pairs and probes can be packaged individually, or can be combined to make a multiple fluorescent PCR detection mixture. In the multiplex fluorescent PCR detection mixture, the amounts of the above-mentioned primers and probes can be conventional amounts known to those skilled in the art.
[0052] That is to say, the kit of the present invention may contain the aforementioned independently packaged sets of primer pairs and probes, or may contain a configured multiplex fluorescent PCR detection mixture containing each set of primer pairs and probes.
[0053] Further, the kit can also contain dNTP, MgCl 2 , PCR buffer, reverse transcriptase, Taq DNA polymerase, sterile wat...
Embodiment 2
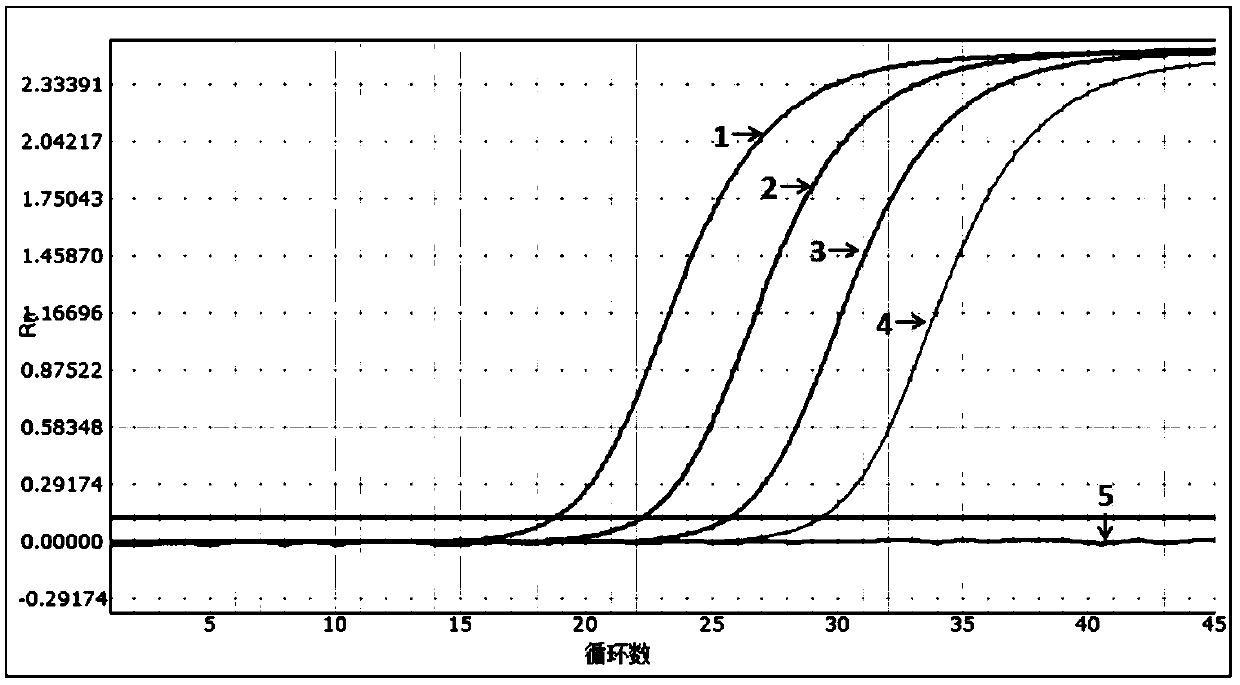
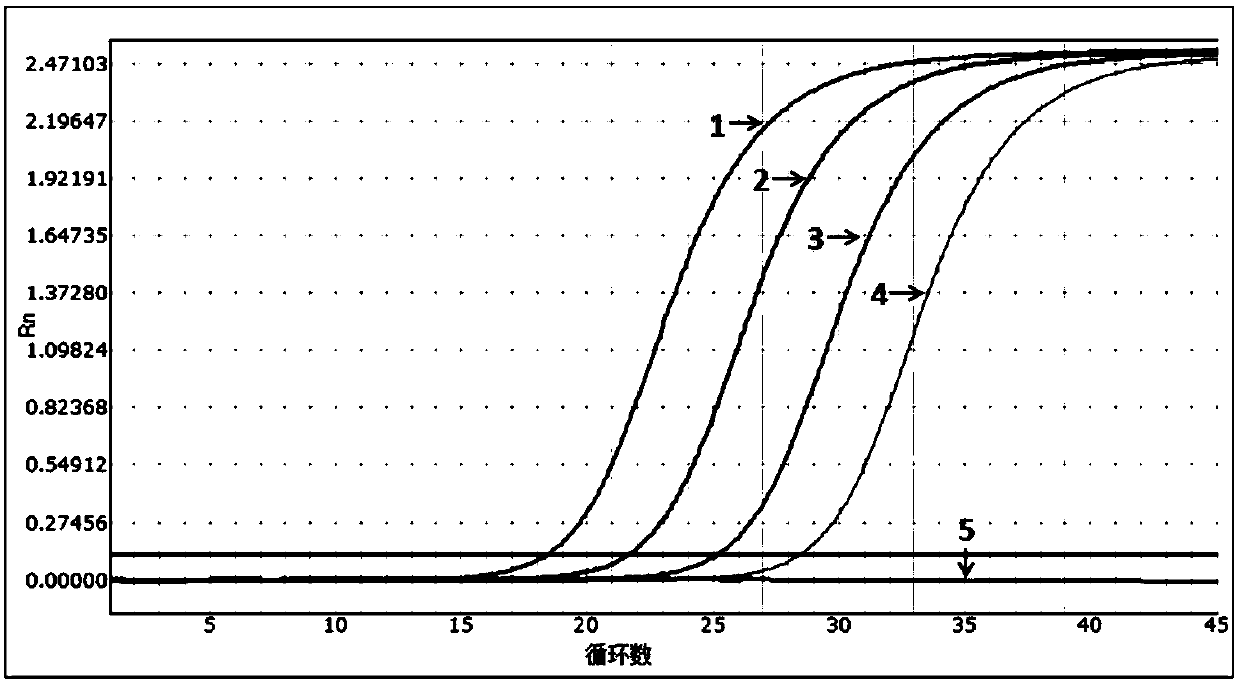
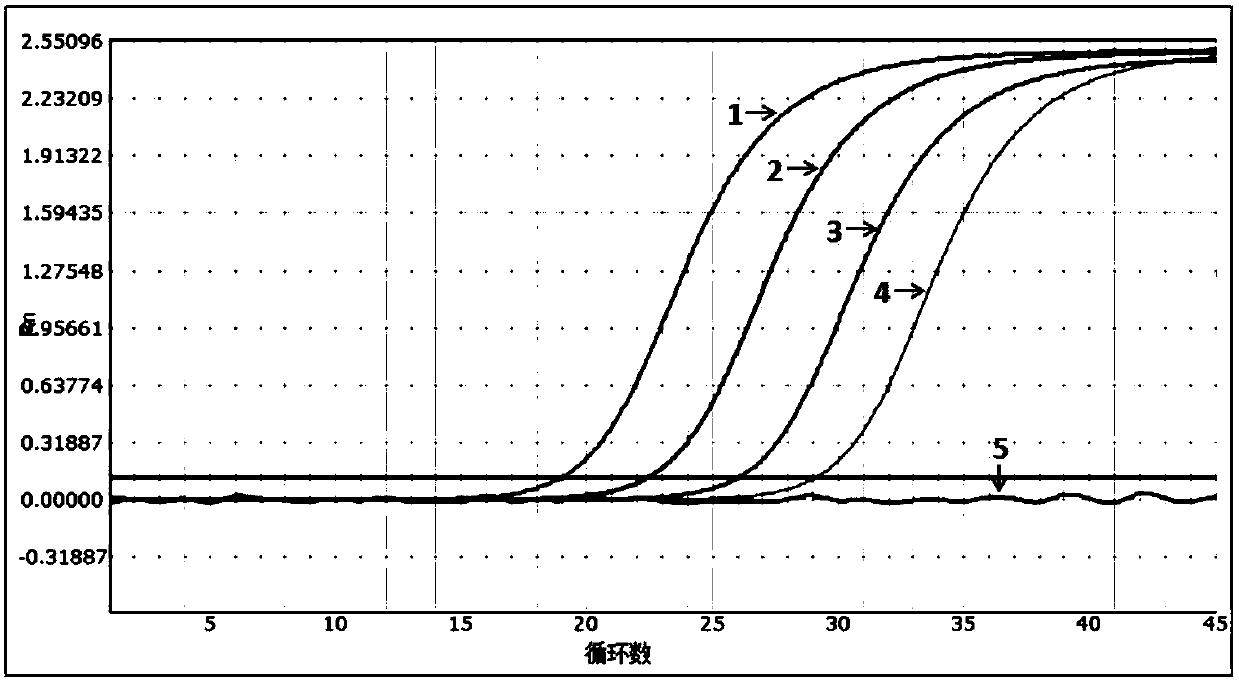
[0054] The sensitivity evaluation of embodiment 2 kit
[0055] Experiment purpose: determine the detection limit (minimum detection concentration) of the present invention (embodiment 1) multiple fluorescent PCR kit
[0056] experimental method:
[0057] 1. Preparation of positive control substance:
[0058] Pseudoviruses containing specific amplified fragments of HIV, HBV and HCV were constructed as positive controls, and the construction method was carried out with reference to "Construction and Expression of Ribonuclease-resistant Virus-Like Particles Containing Long Fragment Chimeric RNA (2008)".
[0059] The nucleic acid sequence of the constructed pseudovirus was verified by Sanger sequencing, and the amplicon fragment containing HIV, HBV and HCV 3 detection primer pairs in Example 1 could be recognized by the aforementioned 3 primers and probes.
[0060] Wherein, the nucleotide sequence of the HIV-specific amplified fragment is shown in SEQ ID NO.10, specifically:
...
Embodiment 3
[0086] Example 3 Evaluation of the detection effect of the kit
[0087] experimental method:
[0088] 1. Sample processing:
[0089] The samples used include: HIV, HBV, HCV patient serum samples of 2 cases each, and healthy human serum samples of 2 cases.
[0090] The above serum samples were all extracted with ribonucleic acid (RNA) extraction kit (magnetic bead column extraction method) produced by Shanghai Zhijiang Biotechnology Co., Ltd.
[0091] The components of the kit for extracting viral RNA by magnetic bead method are shown in Table 5:
[0092] table 5
[0093]
Element
volume / person
1
affinity column
1 tube
2
binding buffer
500μl
3
Washing solution A
1ml
4
Washing liquid W
1ml
5
eluent
50μl
6
magnetic beads
20μl
7
RNA precipitation aid
6μl
[0094] The method of using the magnetic bead method extraction virus RNA kit is as follows:
[0095] ①Prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com