Superparamagnetism functional particles, magnetized red blood cells and clinic application of superparamagnetism functional particles and magnetized red blood cells
A superparamagnetic, red blood cell technology, applied in the field of medical testing, can solve the problem of no disclosure of blood group antibodies, etc., and achieve the effects of a simple serological detection test method, easy dispersion, and uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
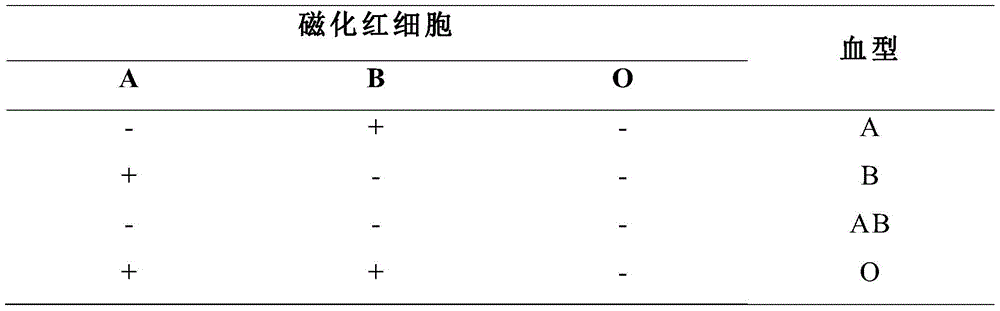
Image
Examples
Embodiment 1
[0050] Embodiment 1 A kind of superparamagnetic functional particle
[0051] The particles are incubated with bacterial magnetic particles and anti-erythrocyte glycoprotein GPA monoclonal antibody after the surface group is activated by the coupling agent:
[0052] The preparation method of the superparamagnetic functional particle is as follows:
[0053] a. Activation: adding a mixture of EDC and NHS with a mass ratio of 1:1 to activate the bacterial magnetic particles to obtain activated bacterial magnetic particles;
[0054] b. Incubation: incubate the activated bacterial magnetic particles with anti-erythrocyte glycoprotein GPA monoclonal antibody at room temperature;
[0055] c. Separation: Magnetic adsorption and washing to obtain superparamagnetic functional particles.
Embodiment 2
[0056] Embodiment 2 A kind of superparamagnetic functional particle
[0057] The particles are incubated with bacterial magnetic particles and anti-erythrocyte glycoprotein GPA monoclonal antibody after the surface group is activated by the coupling agent:
[0058] The preparation method of the superparamagnetic functional particle is as follows:
[0059] a. Activation: adding a mixture of EDC and NHS with a mass ratio of 1:1 to activate the bacterial magnetic particles to obtain activated bacterial magnetic particles;
[0060] b. Incubation: the activated bacterial magnetic particles and the anti-erythrocyte glycoprotein GPA monoclonal antibody are added to an incubation agent and incubated at room temperature; mixture;
[0061] c. Separation: Magnetic adsorption and washing to obtain superparamagnetic functional particles.
Embodiment 3
[0062] Embodiment 3 A kind of superparamagnetic functional particles
[0063] The particles are incubated with bacterial magnetic particles and anti-erythrocyte glycoprotein GPA monoclonal antibody after the surface group is activated by the coupling agent:
[0064] The preparation method of the superparamagnetic functional particle is as follows:
[0065] (1) Take bacterial magnetic particles, add a treatment agent to react under stirring conditions, and wash the product until neutral after the reaction; mixed solution;
[0066] (2) The bacterial magnetic particles obtained after the step (1) reaction is made into a solution with a concentration of 0.1%, and the pH value of the solution is adjusted to 3.0. Adding a concentration of 5% 3-aminopropyl triethoxy to the above solution In the silane ethanol solution, react under stirring conditions, wash the product to neutrality after the reaction is completed, and prepare the treated bacterial magnetic particles;
[0067] (3) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com