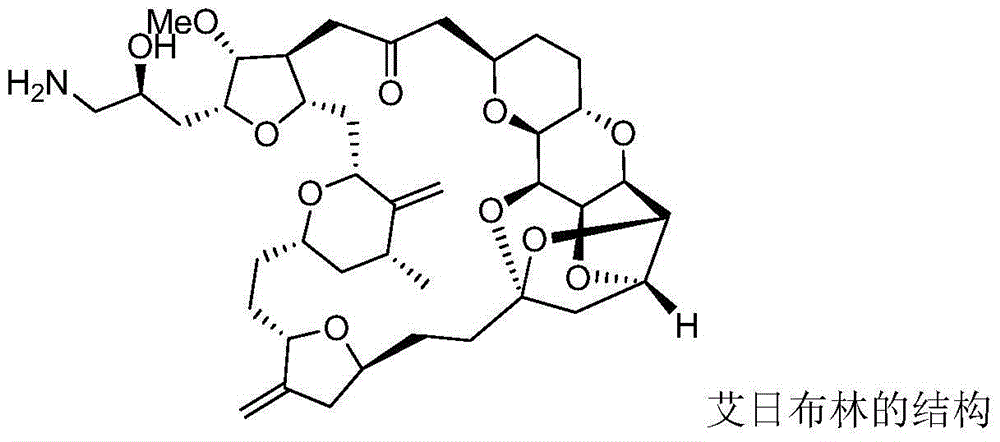
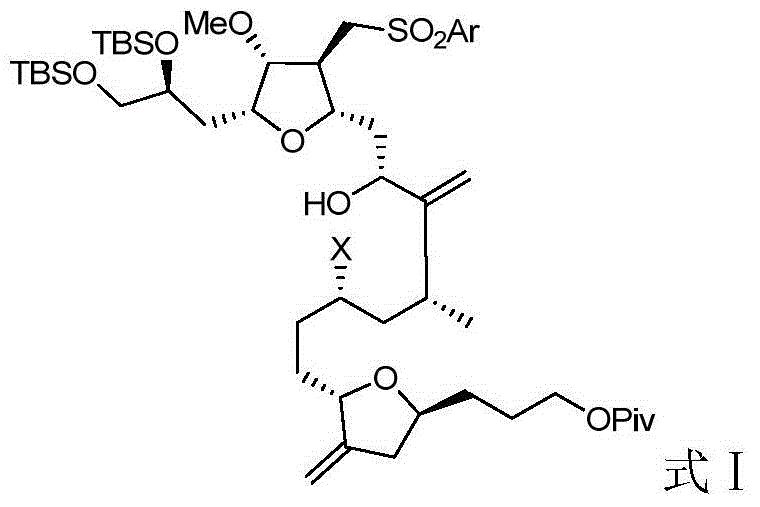
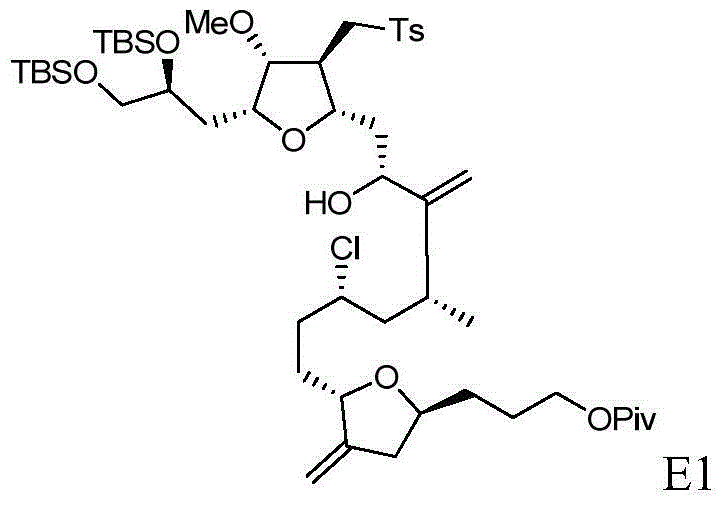
Intermediate for preparing eribulin and preparation method thereof
A compound and selected technology, applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as total synthesis is very difficult, and achieve easy quality control and refractory The effect of less impurities and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 1
[0043] The synthesis of embodiment one F1
[0044] step 1:
[0045]
[0046] Compound F1-1 (1.17g) was dissolved in dry tetrahydrofuran (8ml), added tetrabutylammonium fluoride (563mg), reacted under nitrogen protection, TLC monitored the reaction was complete, added water (10ml), and then added ethyl acetate Extraction (15ml×3), combined organic phases, washed with saturated brine (50ml), dried over anhydrous magnesium sulfate, filtered, and spin-dried to obtain compound F1-2 (730mg).
[0047] MS:413[M+H] + .
[0048] Step 2:
[0049]
[0050] Compound F1-2 (730mg) was dissolved in dry dichloromethane (7ml), cooled to 0°C, added pyridine (419mg), DMAP (14mg) and PivCl (213mg) successively, stirred at room temperature for 1.5 hours under nitrogen protection, and used It was quenched with saturated ammonium chloride solution (10 ml). Then the organic layer was washed with water (10ml), saturated sodium bicarbonate solution (10ml) and saturated brine (10ml), dried ov...
Embodiment 2 2
[0052] The synthesis of embodiment two F2
[0053] step 1:
[0054]
[0055] SM2 (29g, 0.095mol) was dissolved in tetrahydrofuran (90ml), cooled to 10°C, LHMDS solution in THF (1M, 95.2ml) was added dropwise, and stirred at 10°C for half an hour. Subsequently, a toluene solution (140 ml) of compound F2-1 (35 g, 0.068 mol) was added dropwise, and the reaction was continued at 10° C. for half an hour after the drop was completed. The completion of the reaction was monitored by TLC. The reaction solution was washed with 1M aqueous hydrochloric acid (230ml) and saturated brine (175ml) successively, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain crude product F2-2 (60g).
[0056] MS:667[M+H] + .
[0057] Step 2:
[0058]
[0059]The crude compound F2-2 (60g) was dissolved in acetonitrile (200ml) and toluene (200ml), added TMSI (60ml), and reacted at 45°C for 2-4h. TLC monitors that the reaction is complete, the reac...
Embodiment 3 1
[0092] The synthesis of embodiment three E1
[0093]
[0094] Two Schlenk reaction vials (marked as 1 and 2) were pre-dried under an infrared lamp for 2-3 hours, then further dried with an alcohol torch under vacuum (vacuum oil pump), and then cooled to room temperature under vacuum.
[0095] Weigh CrCl in the glove box 2 (148mg), Liagnd4 (583mg), protonsponge (282mg) into Schlenk reaction flask 1, weigh Mn powder (220mg), NiCl 2 dmp (68mg), Compound F1 (992mg), Compound F2 (1.47g), LiCl (168mg), ZrCp 2 Cl 2 (876mg) was added to Schlenk reaction flask 2, and then the two reaction flasks were taken out, replaced in vacuum-argon 3 times, and then added CH 3 CN (5ml) to Schlenk reaction bottle 1, stirring reaction for 1-2 hours; Schlenk reaction bottle 2 is stand-by under the protection of argon;
[0096] The solution in the Schlenk reaction bottle 1 was transferred to the Schlenk reaction bottle 2 under the action of vacuum-argon with a double-ended needle, and then sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com