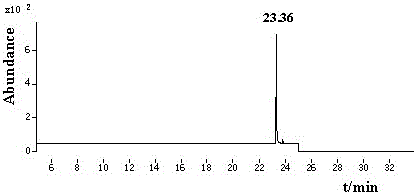
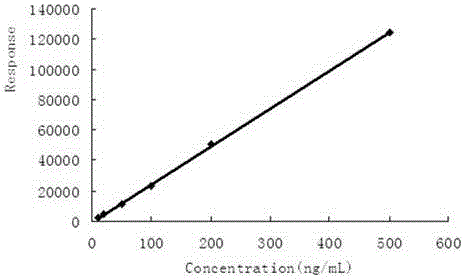
GC-NIC-MS measurement method for penflufen residual amount
A GC-NCI-MS and flufenapyroxamide technology, which is applied in the field of GC-NCI-MS determination of flufenapyroxad residues, can solve the problems of low sensitivity of anti-interference ability, avoid matrix interference, and have good repeatability , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Detection of flufenapyramide residues in wheat
[0035] (1) Sample pretreatment
[0036] extract
[0037] Weigh 5g of wheat sample (ground into flour) that has been thoroughly mixed into a 50mL centrifuge tube, add 20mL of water to resuscitate for 30min, then accurately add 20mL of acetonitrile solution containing 1% acetic acid, shake and extract for 20min, ultrasonically extract for 5min, add 3g of anhydrous Magnesium sulfate and 2g sodium acetate, after vortexing for 1min, centrifuge at 7000r / min for 5min. After centrifugation, take 8 mL of acetonitrile extract and rotate it at 40 °C or blow it with nitrogen until it is nearly dry, add 1 mL of acetonitrile and vortex it, and wait for purification.
[0038] purify
[0039] Pre-wash C with 5 mL of acetonitrile 18 / PSA solid phase extraction column, when the liquid level reaches the top of the adsorbent, transfer the above extraction solution into the column, wash the test tube with 2mL acetonitrile, and ...
Embodiment 2
[0065] Example 2: Detection of flufenapyroxamide residues in pork
[0066] (1) Sample pretreatment
[0067] extract
[0068] Weigh 5g of fully mixed pork sample into a 50mL centrifuge tube, add 5mL of water to mix, let it stand for 30min, add 20mL of acetonitrile accurately, extract homogeneously for 2min, add 3g of anhydrous magnesium sulfate and 2g of sodium chloride, and vortex for 1min , 7000r / min centrifugal 5min. After centrifugation, take 8 mL of the acetonitrile extract at 40°C for rotary steaming or nitrogen blowing to about 1 mL for purification.
[0069] purify
[0070] Pre-wash C with 5 mL of acetonitrile 18 / PSA solid phase extraction column, when the liquid level reaches the top of the adsorbent, transfer the above extraction solution into the column, wash the test tube with 2mL acetonitrile, and transfer the washing liquid into the SPE column, when the solution reaches the top of the adsorbent, add Add 4mL of acetonitrile to the column for elution, all the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com