Lipoprotein phospholipase A2 detection kit
A detection kit and lipoprotein technology, applied in the field of medical detection, can solve the problems of inability to accurately quantitatively measure, falsely increase the detection value, and use a large amount of antibodies, so as to reduce the amount of antibodies, improve accuracy, and reduce production. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 lipoprotein phospholipase A2 detection kit
[0022] A. Preparation of reagent R1:
[0023] Accurately weigh 5g PEG8000, 10g sodium chloride, 8g sucrose, 5g Tween 20 and 1g sodium azide; dissolve in 1L of 0.25M ammonium chloride buffer, adjust with 1mol / L hydrochloric acid and 1mol / L sodium hydroxide solution The pH value of the solution is 9.0.
[0024] B. Preparation of reagent R2:
[0025] Anti-lipoprotein phospholipase A2 antibody: mouse monoclonal antibody, titer determined by ELISA method is 1:50000.
[0026] Latex particles: use polystyrene latex particles with a particle size of 150-200nm with carboxyl groups.
[0027] 1. Take 1ml (50mg / ml) latex particles, wash with 0.2M, pH5.0 MES solution (2-morpholineethanesulfonic acid buffer) three times, and disperse;
[0028] 2. Add 0.05ml of 10mg / ml EDAC solution freshly prepared with 0.2M, pH5.0 MES solution, and react at room temperature for 1 hour;
[0029] 3. Add 0.5ml of 100mg / ml...
Embodiment 2
[0038] Embodiment 2 kit performance investigation
[0039] Adopt the kit prepared in embodiment 1 to carry out following test:
[0040] 1. Precision test
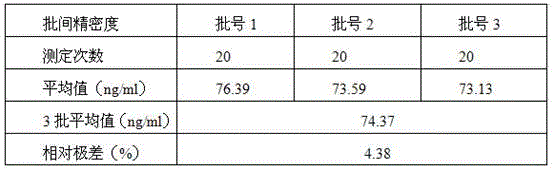
[0041] Two human serum samples with different lipoprotein phospholipase A2 contents were used to determine the intra-assay precision of the detection kit of the present invention. Three batches of detection kits were used to measure the same human serum sample 20 times respectively, and the inter-batch relative range of the detection kit of the present invention was calculated. The results showed that the intra-assay precision of the detection kit of the present invention was 4.26% and 1.78% (see Table 1), and the inter-assay relative range was 4.38% (see Table 2).
[0042] Table 1
[0043]
[0044] Table 2
[0045]
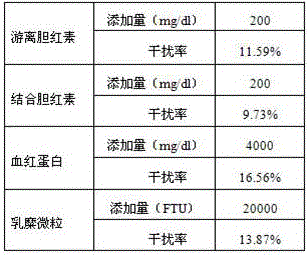
[0046]2. Linearity measurement test
[0047] Determination of different lipoprotein phospholipase A2 protein content standards, the concentrations of lipoprotein phospholipase A2 protein standards w...
Embodiment 3
[0054] Embodiment 3 test kit stability investigation
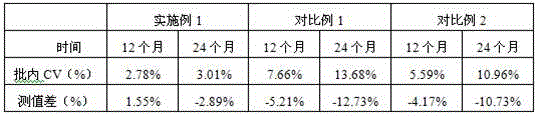
[0055] Under the storage condition of 2-8°C, the same serum sample was measured at 0 month, 12 month and 24 month respectively, each sample was measured 20 times, and the average value was taken (see Table 4 for test results). The results show that the difference in measured values is very small, indicating that the detection kit prepared in Example 1 can be stable for at least 24 months under storage conditions of 2-8 degrees.
[0056] Table 4
[0057]
[0058] Measured value difference = (measured value - measured value at 0 months) / measured value at 0 months × 100%
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com