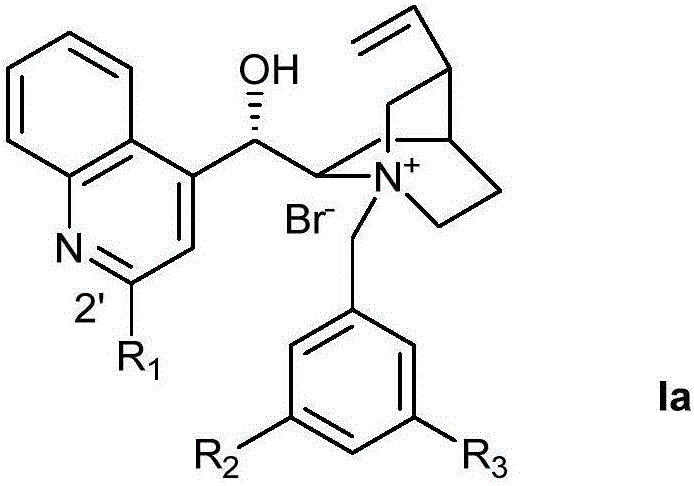
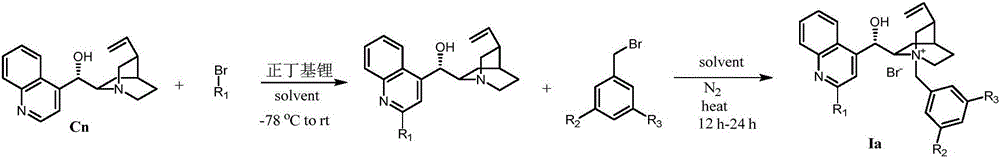
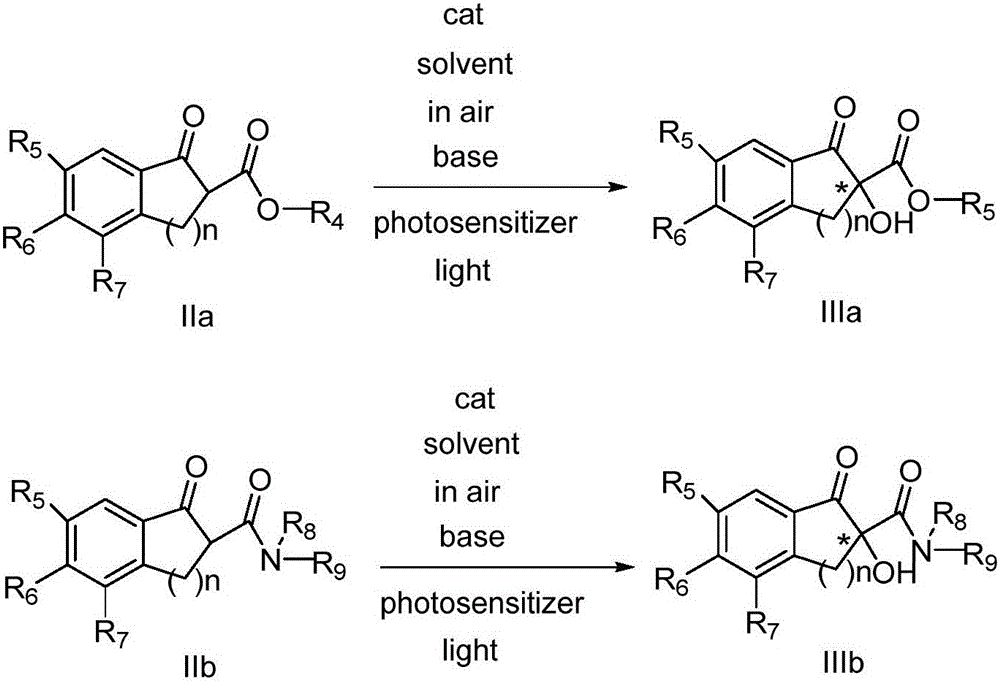
Novel method for asymmetric alpha-hydroxylation of photo-oxygenation beta-dicarbonyl compound based on C-2' phase transfer catalyst
A technology of dicarbonyl compound and phase transfer catalyst, applied in the field of organic synthesis, can solve the problems of harsh requirements and low catalytic activity, and achieve the effects of high catalytic activity, good substrate applicability and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Ia-1 (Ia, R 1 is phenyl, R 2 for Br, R 3 for Br)
[0031]
[0032] Add 10ml of ether into a 100ml three-necked flask, stir 4.0g of bromobenzene, slowly add 10.4ml of n-butyllithium, react at -10°C for two hours, then add dropwise 50ml of cinchonine (3.0g) solution dissolved in ether, continue Stir for an hour. After warming to room temperature, the reaction was stirred for an additional two hours until complete. The reaction was quenched with acetic acid, and 50ml of water and 50ml of ethyl acetate were added. Subsequently, 2.5 g of elemental iodine was added until all solids dissolved, followed by 1.0 g of Na 2 S 2 o 5 , the mixture was adjusted to alkaline with 20% ammonia water. The organic phase was washed with water, dried over anhydrous sodium sulfate, and spin-dried. Crude product column chromatography (15% MeOH, 2% Et 3 N, 40% EtOAcinPE), followed by recrystallization from ethyl acetate to give 0.80 g of white solid (Cn-1). 1 HNMR (5...
Embodiment 2
[0034] Preparation of Ia-2 (Ia, R 1 is 4-trifluoromethylphenyl, R 2 for Br, R 3 for Br)
[0035]
[0036] In a 100ml three-necked flask, add 10ml of ether, stir 5.5g of 4-trifluoromethylbromobenzene, slowly add 10.4ml of n-butyllithium, react at -10°C for two hours, then dropwise add 50ml of diethyl ether-dissolved cinchonine (3.0 g) solution, stirring was continued for one hour. After warming to room temperature, the reaction was stirred for an additional two hours until complete. The reaction was quenched with acetic acid, and 50ml of water and 50ml of ethyl acetate were added. Subsequently, 2.5 g of elemental iodine was added until all solids dissolved, followed by 1.0 g of Na 2 S 2 o 5 , the mixture was adjusted to alkaline with 20% ammonia water. The organic phase was washed with water, dried over anhydrous sodium sulfate, and spin-dried. Crude product column chromatography (15% MeOH, 2% Et 3 N, 40% EtOAcinPE), followed by recrystallization from ethyl acetate...
Embodiment 3
[0038] Preparation of Ia-3 (Ia, R 1 is 4-trifluoromethylphenyl, R 2 for CF 3 , R 3 for CF 3 )
[0039]
[0040] Into a 100ml three-necked flask, add 0.24g of Cn-2, 20ml of tetrahydrofuran, and 0.31g of 3,5-trifluoromethylbenzyl bromide. The reaction was heated to reflux for 10 hours. After the reaction was completed, it was cooled to room temperature, poured into 50ml of diethyl ether, filtered, and the solid was recrystallized from methanol / diethyl ether to obtain 0.31g of Ia-3 with a yield of 76%. 1 HNMR (500MHz, Methanol-d 4 )δ8.59–8.38(m,6H),8.32–8.20(m,2H),7.97–7.79(m,4H),6.69(d,J=2.6Hz,1H),6.13(m,1H),5.48 –5.39(m,1H),5.38–5.24(m,3H),4.58(ddd,J=11.7,8.4,2.8Hz,1H),4.12(dt,J=34.5,10.1Hz,2H),3.61(t ,J=11.3Hz,1H),3.23–3.11(m,1H),2.78–2.53(m,2H),2.07–1.84(m,3H),1.21(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com