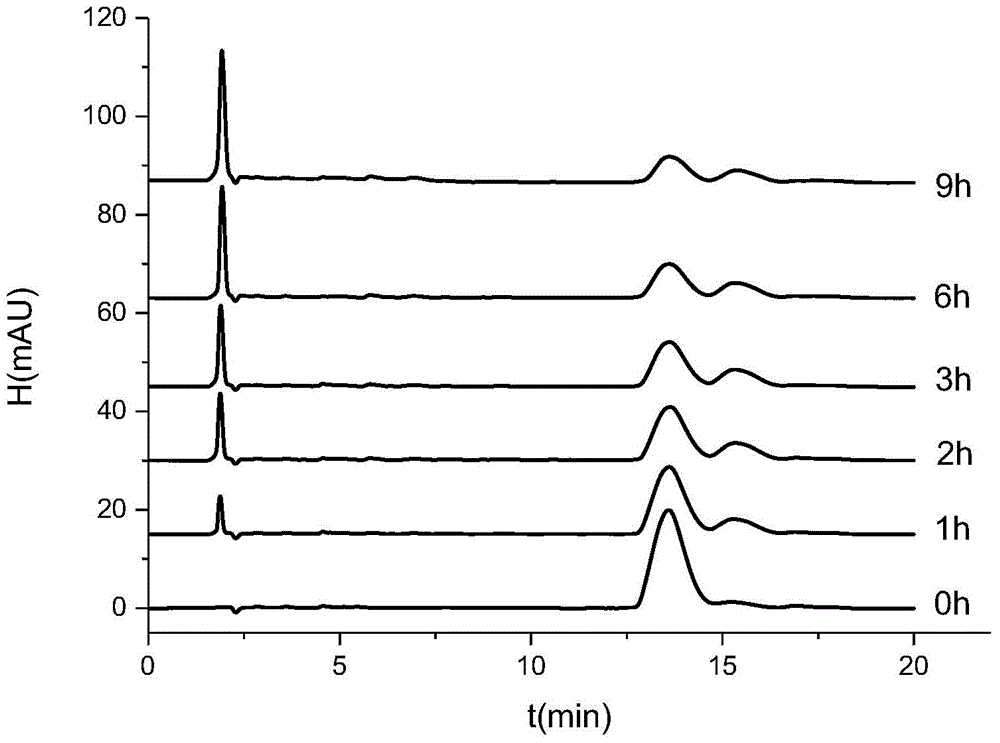
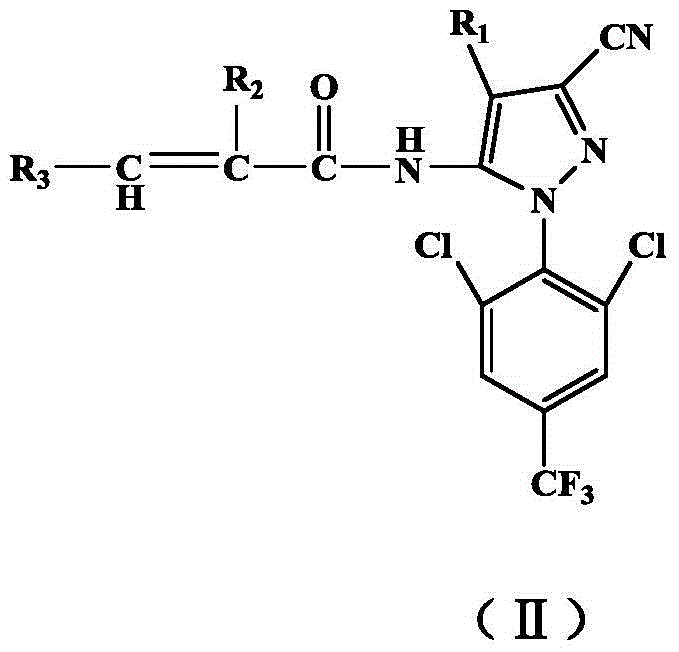
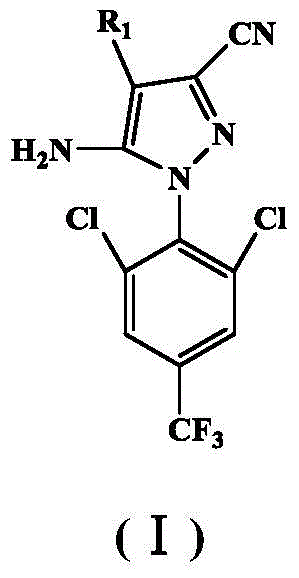
Acrylamidoaryl pyrazole compounds, and one-pot synthesis method and application thereof
An acrylamide-based compound technology, used in applications, animal repellants, plant growth regulators, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1, 3-cyano-4-trifluoromethylsulfinyl-5-acrylamido-1-(2,6-dichloro-4-trifluoromethylphenyl)-pyrazole ((II ) Synthesis of 1)
[0050]
[0051] Add 25mL tetrahydrofuran and 4.5g 1-(2,6-dichloro-4-trifluoromethyl)phenyl-3-cyano-4-trifluoromethylsulfinyl-5-aminopyrazole into a 100mL reactor , 1g of sodium hydroxide, 0.96g of acryloyl chloride, after mixing evenly, put the reactor into an oven, the reaction temperature is 90°C, and the reaction time is 720 minutes. After the reaction was completed, the solvent was removed by rotary evaporation, the pH was adjusted to neutral, extracted, dried over anhydrous magnesium sulfate, and passed through the column with petroleum ether and ethyl acetate (4:1) to obtain 4.42 g of (II) 1 as a light yellow solid. Yield is 88.2%.mp:180.1~183.6℃.IRν(cm -1 ): 3197 (N—H), 3073 (C—H), 2251 (—CN), 1716 (—C=O), 1631 (pyrazole ring skeleton vibration), 1535 and 1405 (benzene ring skeleton vibration), 1315 (C—F), 882 (aromatic ring C...
Embodiment 2
[0052] Example 2, 3-cyano-4-trifluoromethylsulfinyl-5-(2-methylacryl)amido-1-(2,6-dichloro-4-trifluoromethylphenyl) -Synthesis of pyrazole ((Ⅱ)2)
[0053]
[0054] Add 25mL dioxane, 4.5g 1-(2,6-dichloro-4-trifluoromethyl)phenyl-3-cyano-4-trifluoromethylsulfinyl-5- Aminopyrazole, 2.65g of sodium carbonate, 1.13g of 2-methacryloyl chloride, after mixing evenly, put the reactor into an oven, the reaction temperature is 110°C, and the reaction time is 720 minutes. After the reaction was completed, the solvent was removed by rotary evaporation, the pH was adjusted to neutral, extracted, dried over anhydrous magnesium sulfate, and passed through the column with petroleum ether and ethyl acetate (4:1) to obtain 4.28 g of (II) 2 as a light yellow solid. Yield is 82.3%.mp:162.4~166.4℃.IRν(cm -1 ): 3364 (N—H), 3073 (C—H), 2255 (—CN), 1702 (—C=O), 1633 (pyrazole ring skeleton vibration), 1550 and 1409 (benzene ring skeleton vibration), 1316 (C—F), 877 (aromatic ring C—H). 1 HNMR (...
Embodiment 3
[0055] Example 3, 3-cyano-4-trifluoromethylsulfinyl-5-(3-methylacryl)amido-1-(2,6-dichloro-4-trifluoromethylphenyl) -Synthesis of pyrazole ((Ⅱ)3)
[0056]
[0057] Add 25mL of toluene and 4.5g of 1-(2,6-dichloro-4-trifluoromethyl)phenyl-3-cyano-4-trifluoromethylsulfinyl-5-aminopyrazole into a 100mL reactor , 1.7g sodium ethoxide, and 1.15g 3-methacryloyl chloride, after mixing evenly, put the reaction kettle into an oven, the reaction temperature is 120°C, and the reaction time is 720 minutes. After the reaction, remove the solvent by rotary evaporation, adjust the pH to neutral, extract, dry over anhydrous magnesium sulfate, and pass through the column with petroleum ether and ethyl acetate (4:1) to obtain 4.32 g of (II) 3 as a yellow solid. The rate is 85.5%.mp:172.4~175℃.IRν(cm -1 ): 3416 (N—H), 3084 (C—H), 2252 (—CN), 1710 (—C=O), 1647 (pyrazole ring skeleton vibration), 1540 and 1382 (benzene ring skeleton vibration), 1312 (C—F), 880 (aromatic ring C—H). 1 HNMR (CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com