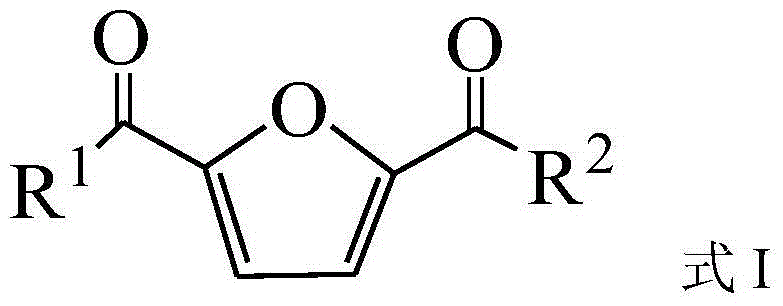
Preparation method of 2,5-diacyl furan compound
A technology of diacylfuran and compound is applied in the field of preparation of 2,5-diacylfuran compound, can solve the problems of difficulty in large-scale industrial application, complicated synthesis route, low total yield and the like, and achieves simple and efficient preparation method, The effect of high purity and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
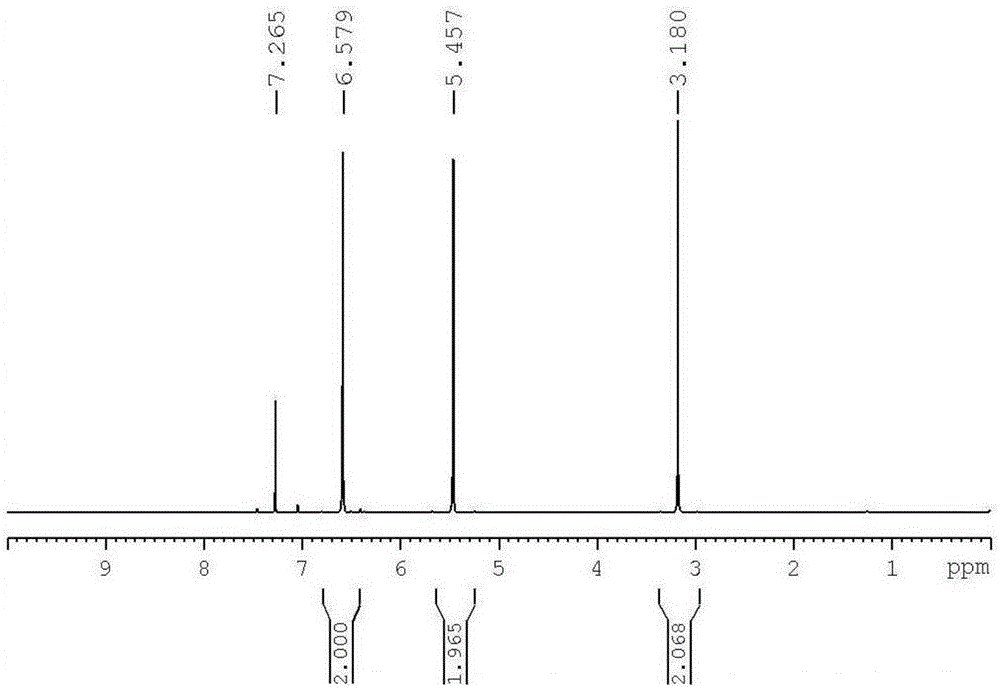
[0053] In a 500ml reactor, add 68.0g furan, 78.5g maleic anhydride, 100ml toluene, react at 50°C for 10h, cool and crystallize, and dry to obtain 2,3-dicarboxylic anhydride-7-oxabicyclo[2.2.1 ]hept-5-ene monomer, the product is a white crystal, and the yield is 92%. Obtained by 1H-NMR (400MHz, CDCl3), there are three peaks of CH and 2H on the ring, which are respectively δ (3.18, 5.46, 6.58), such as figure 1 shown.
[0054] Take 16.6g of 2,3-dicarboxylic anhydride-7-oxabicyclo[2.2.1]hept-5-ene monomer into a 100ml reactor, add 20.4g of acetic anhydride, and react with 0.02mol concentrated sulfuric acid at 160°C for 2h After the reaction was completed, it was lowered to room temperature, acetic anhydride was distilled off under reduced pressure, and sublimed to obtain white crystal 2,5-diacetylfuran, the yield was 86%, and the melting point was 135-136 ° C. Liquid phase mass spectrometry (LC-MS) The measured molecular weight is 152.1, and the 1H-NMR (400MHz, CDCl3) test is o...
Embodiment 2
[0056] In a 500ml reactor, add 68.0g furan, 19.6g maleic anhydride, 20ml dichloromethane, react at 100°C for 1h, cool and crystallize, and dry to obtain 2,3-dicarboxylic anhydride-7-oxabicyclo[2.2 .1] Hept-5-ene monomer, the product is white crystal, the yield is 90%. through 1 H-NMR (400MHz, CDCl 3 ) test, CH and 2H on the ring have three peaks, which are respectively δ (3.18, 5.46, 6.58).
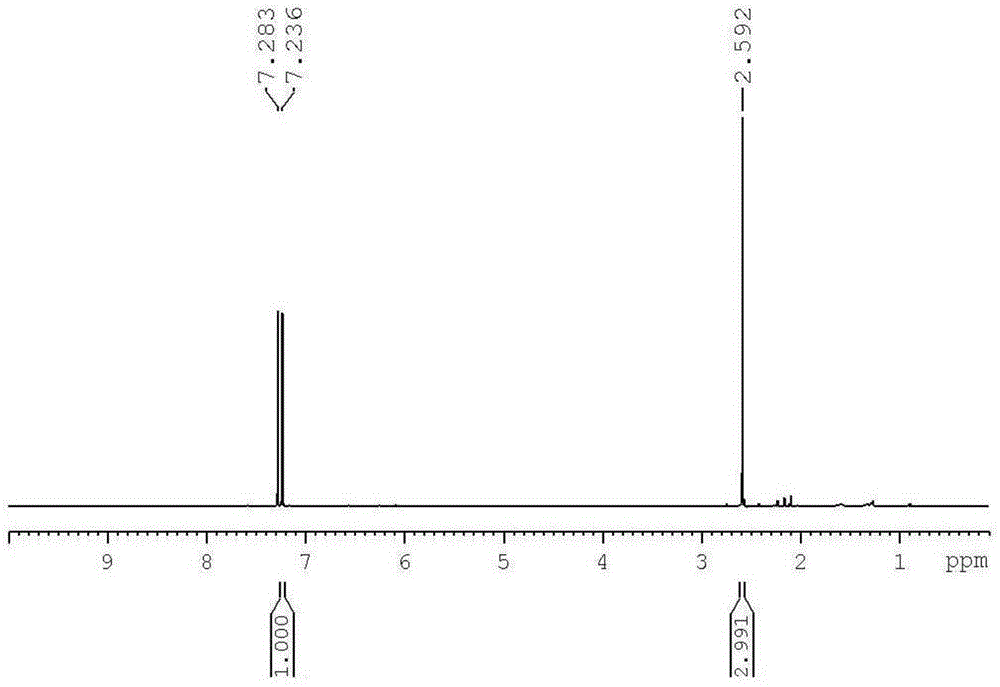
[0057] Take 16.6g of 2,3-dicarboxylic anhydride-7-oxabicyclo[2.2.1]hept-5-ene monomer and put it into a 250ml reactor, add 102.6g of chloroacetic anhydride, and react with 0.025mol concentrated nitric acid at 200°C 0.5h, after the reaction was completed, it was lowered to room temperature, and chloroacetic anhydride was distilled off under reduced pressure, and sublimated to obtain white crystal 2,5-dichloroacetylfuran, with a yield of 93%, as measured by liquid phase mass spectrometry (LC-MS). Molecular weight 221.0, 1 H-NMR (400MHz, CDCl 3 ) test, CH on the furan ring, 2H, δ (7.24)...
Embodiment 3
[0059] In a 500ml reactor, add 68.0g furan, 9.8g maleic anhydride, 10ml chloroform, react at 70°C for 2h, cool and crystallize, and dry to obtain 2,3-dicarboxylic anhydride-7-oxabicyclo[2.2 .1] Hept-5-ene monomer, the product is white crystal, the yield is 96%. through 1 H-NMR (400MHz, CDCl 3 ) test, CH and 2H on the ring have three peaks, which are respectively δ (3.18, 5.46, 6.58).
[0060] Take 8.3g of 2,3-dicarboxylic anhydride-7-oxabicyclo[2.2.1]hept-5-ene monomer and add it to a 250ml reactor, add 57.8g trifluoroacetic anhydride, 0.005mol concentrated hydrochloric acid at 160°C Reacted for 6h, after the reaction was completed, it was lowered to room temperature, trifluoroacetic anhydride was distilled off under reduced pressure, and white crystal 2,5-bis-(trifluoroacetyl)furan was obtained by sublimation, with a yield of 88%, liquid phase mass spectrometry (LC -MS) records molecular weight 260.1, 1 H-NMR (400MHz, CDCl 3 ) test, CH on the furan ring, 2H, δ (8.14).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com