Phosphorus-containing fluorine-containing synergic flame-retardant compound and preparation method thereof
A technology for synergistic flame retardant and compound, which is applied in the field of phosphorus-containing and fluorine-containing synergistic flame retardant compounds and their preparation to achieve the effects of reducing surface energy, mild reaction conditions and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step 1, the synthesis of the intermediate containing carbon-nitrogen double bond
[0030] First, 2g (3.86mmol) of 2,2-bis[4-(4-aminophenoxy)phenyl] 1,1,1,3,3,3-hexafluoropropane and 1.86g (7.68mmol) Add 3,5-bis(trifluoromethyl)benzaldehyde into a 100mL single-necked flask, dissolve it with 20mL methanol, stir and react at 55°C for 8h, spin the solvent to dryness with a rotary evaporator, and obtain a light yellow powder after vacuum drying solid, which is an intermediate containing a carbon-nitrogen double bond.
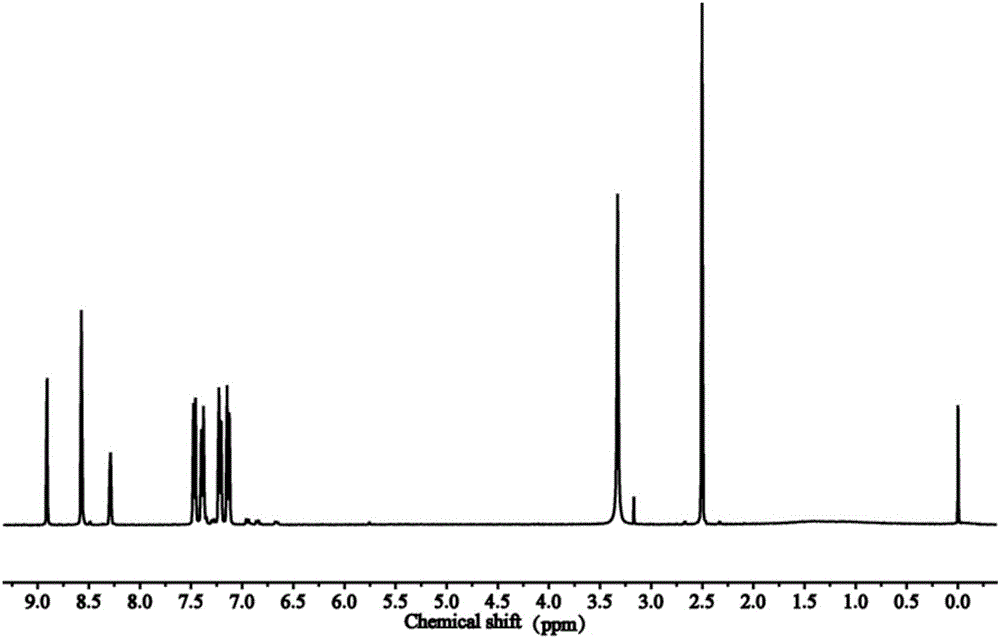
[0031] The characterization of the carbon-nitrogen-containing double bond intermediate is as follows figure 1 As shown, the chemical shifts from 8.8ppm to 9.0ppm are the characteristic peaks of C-H on the carbon-nitrogen double bond, and the six peaks from 7.0ppm to 8.7ppm are the characteristic peaks of C-H on the benzene ring.
[0032] Step 2, synthesis of phosphorus-containing and fluorine-containing synergistic flame-retardant compounds:
[0033] Add 2g...
Embodiment 2
[0036] Step 1, the synthesis of the intermediate containing carbon-nitrogen double bond:
[0037] First, 2g (3.86mmol) of 2,2-bis[4-(4-aminophenoxy)phenyl] 1,1,1,3,3,3-hexafluoropropane and 1.86g (7.68mmol) Add 3,5-bis(trifluoromethyl)benzaldehyde into a 100mL single-necked flask, dissolve in 20mL methanol, stir and react at 65°C for 6h, spin the solvent to dryness with a rotary evaporator, and obtain a light yellow powder after vacuum drying solid, which is an intermediate containing a carbon-nitrogen double bond.
[0038] Step 2, synthesis of phosphorus-containing and fluorine-containing synergistic flame-retardant compounds:
[0039]Add 2g (2.1mmol) of the carbon-nitrogen double bond intermediate obtained in step 1 and 0.91g (4.2mmol) of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide into a 100mL single port In the flask, dissolve with 20mL N,N-dimethylformamide, react at 45°C for 8 hours, precipitate with methanol, wash repeatedly three times, and obtain a light yell...
Embodiment 3
[0041] Step 1, the synthesis of the intermediate containing carbon-nitrogen double bond:
[0042] First, 2g (10.08mmol) of 4,4'-diaminodiphenylmethane and 4.88g (20.16mol) of 3,5-bis(trifluoromethyl)benzaldehyde were added to a 100mL single-necked flask, and 20mL of methanol Dissolve, stir and react at 55° C. for 8 h, spin the solvent to dryness with a rotary evaporator, and obtain a light yellow powdery solid after vacuum drying, which is an intermediate containing a carbon-nitrogen double bond.
[0043] Step 2, synthesis of phosphorus-containing and fluorine-containing synergistic flame-retardant compounds:
[0044] Add 2g (3.10mmol) of the carbon-nitrogen double bond intermediate obtained in step 1 and 1.34g (6.19mmol) of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide into a 100mL single port In the flask, dissolve with 20mL N,N-dimethylformamide, react at 55°C for 10h, precipitate with methanol, wash repeatedly three times, and obtain a light yellow powdery solid afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com