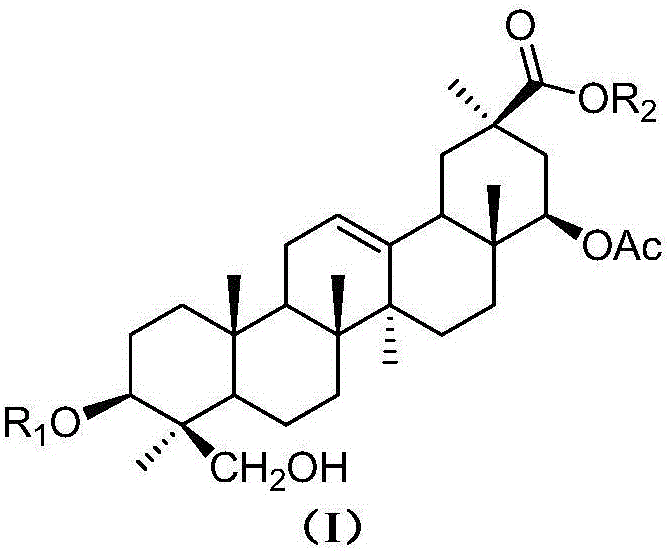
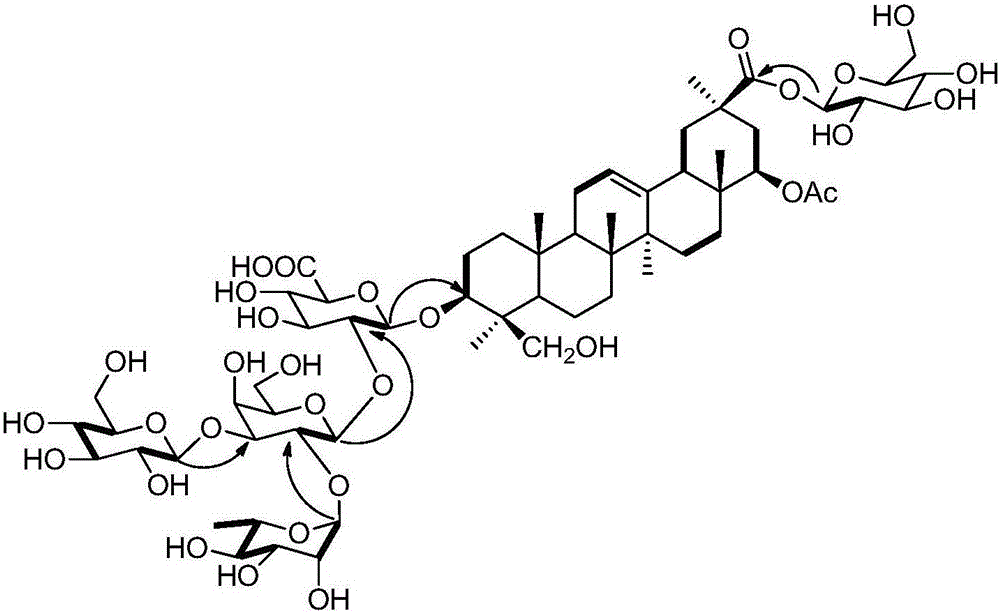
Natural sweeteners oleanane-type triterpenoid saponin compounds as well as preparation methods and application thereof
A kind of technology of triterpenoid saponins and oleanane type, applied in the field of sweetener and natural medicinal chemistry, can solve the problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
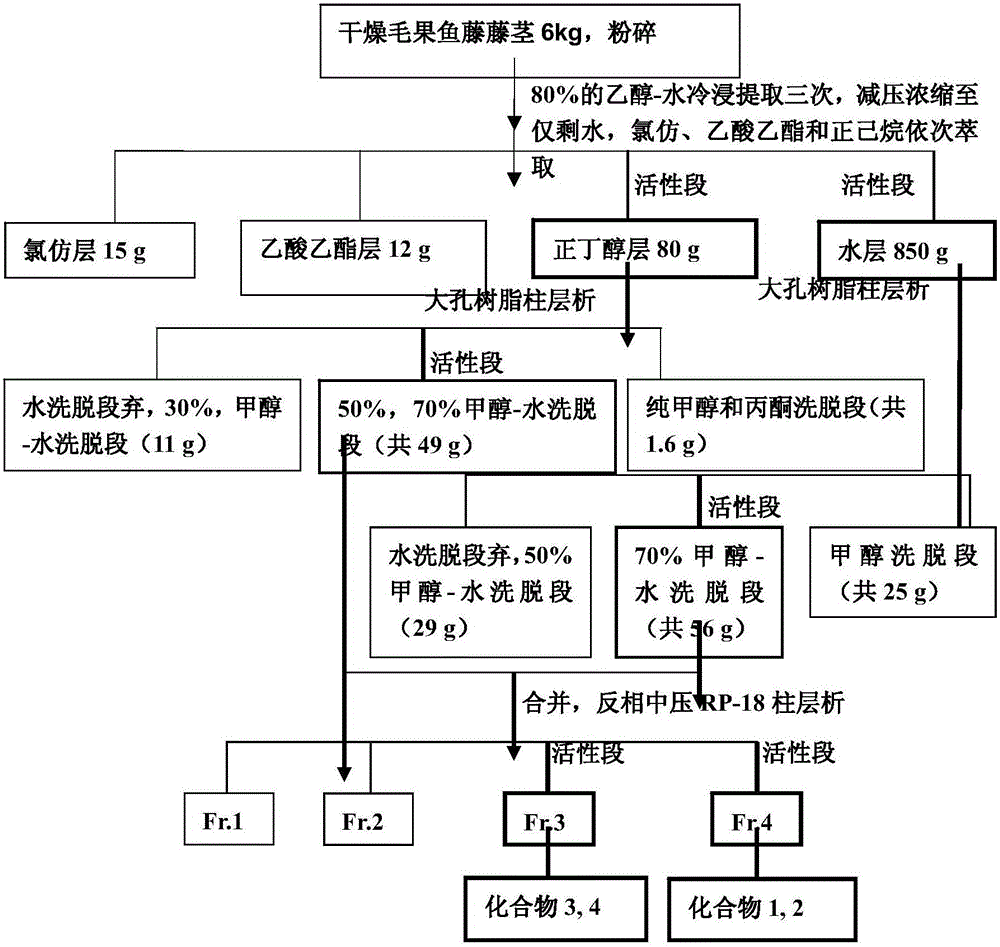
[0039] Extraction, separation and purification of the sweet total saponins extract of Deeris trichocarpa and compounds 1-4 of the present invention:
[0040] Instruments and materials:
[0041] Specific rotation was measured by HoribaSEPA‐300 spectrophotometer, UV was measured by ShimadzuUV‐2401A UV spectrometer, IR was determined by BruckerTenor‐27 infrared spectrometer, ESI‐MS and MS‐MS were determined by BrukerHCT / Esquire mass spectrometer, NMR Measured by Bruker AM-400, DRX-500, AvanceⅢ600 nuclear magnetic resonance instrument, TMS as internal standard, the unit of chemical shift value δ is ppm, and the unit of coupling constant J is Hz.
[0042] Various types of silica gel column chromatography columns, reverse-phase medium pressure columns, rotary evaporators (BüCHI and EYELA companies), dark box UV analyzers (Shanghai Jiapeng Technology Company), medium pressure liquid chromatography (BüCHI), oil Bath thermostat.
[0043] Normal-phase silica gel plate (Qingdao Ocean C...
Embodiment 2
[0050] Physical and spectral data of compound 1-4 of the present invention:
[0051] MillettiasaponinA (1): white powder, 1 HNMR and 13 CNMR data are shown in Table 1 and Table 2; ESI-MS + (m / z1015,[M+H] + ;1037,[M+Na] + ;1059, [M+2Na‐H] + ; 1075, [M+Na+K‐H] + ), ESI‐MS ‐ (m / z1013,[M-H] ‐ ).
[0052] DerrisaponinA (2): white powder, UV(H 2 O)λ max (nm)(logε):190(3.74); IR(KBr)ν max cm ‐1 :3423(OH),2972,2934,1713,1615,1265,1074,1048; 1 HNMR and 13 See Table 1 and Table 2 for CNMRdata; ESI‐MSm / z1175[M‐H] ‐ ;HR‐EI‐MSm / z1175.5480,[M] ‐ (C 56 h 88 o 26 The calculated value is 1175.5480).
[0053] DerrisaponinB (3): white powder, UV(H 2 O)λ max (nm)(logε):190(3.77); IR(KBr)ν max cm ‐1 :3424(OH),2969,2932,1718,1615,183,1261,1072,1048; 1 HNMR and 13 See Table 1 and Table 2 for CNMR data; ESI‐MSm / z1175[M‐H] ‐ ,1013[M‐162] ‐ ;HR‐EI‐MSm / z1175.5482,[M] ‐ (C 56 h 88 o 26 The calculated value is 1175.5482).
[0054] Derrisaponin C (4): white powder, UV(...
Embodiment 3
[0065] Taste threshold and sweetness test of oleanane-type triterpene saponins 1-4 of the present invention:
[0066]Sweetness evaluation of a single compound: prepare sucrose reference solution gradients with concentrations of 4%, 2%, and 1%, and sample solution gradients with concentrations of 0.02%, 0.01%, 0.005%, 0.0033%, 0.0025%, and 0.002%. The sweetness threshold and sweetness of the samples were evaluated by the evaluation team members. The threshold value refers to the lowest concentration that can taste sweetness (unit: mg / ml); the sweetness refers to the multiple of the sweetness of the sample to the sweetness of sucrose, which is found by comparing the sweetness of a certain concentration of sample with a certain concentration of sucrose The sweetness is equivalent, thereby converting the sweetness of the sample. The taste threshold and sweetness of oleanane-type triterpene saponins 1-4 are shown in Table 3.
[0067] Table 3 Sweetness evaluation of compounds 1-4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com