A kind of lyoprotectant and its application in lyophilized live attenuated hepatitis A vaccine
A technology of freeze-dried protective agent and live attenuated vaccine, which is applied in the field of biological products, can solve the problems of long preparation time and slow dissolution speed of freeze-dried protective agent, and achieve improved dissolution speed, good clinical safety and immunogenicity, Recipe Simple Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
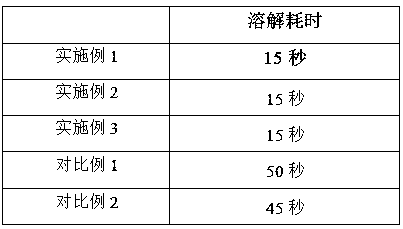
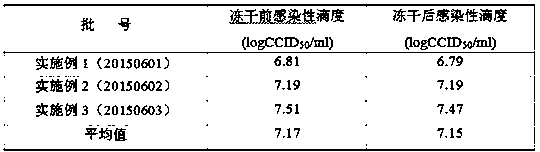
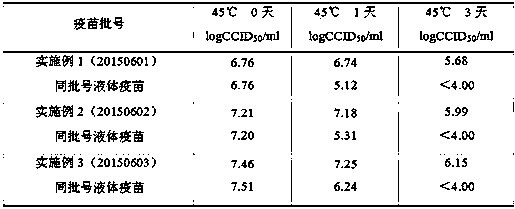
Examples
Embodiment 1
[0027] A. Human embryonic lung diploid cells KMB 17 For passage 30, use 0.04% trypsin + 0.03% ethylenediaminetetraacetic acid to disperse and suspend the monolayer cells in the culture medium at a speed of 8-12 rpm and a temperature of 37±0.5°C Culture for 3-6 days to make the cells grow into a dense monolayer;
[0028] B. KMB that will grow into a dense monolayer 17 Discard the nutrient solution for the cells, and after digestion, collect the cells with MEM medium containing 3% serum, and add live attenuated hepatitis A vaccine virus seeds to the cells according to the ratio of the number of viruses to the number of cells at 1:40-60 , Stir for 1 hour at 37°C with a speed of 100 rpm to suspend and adsorb the virus, add maintenance solution containing 8% serum to the final volume of each spinner bottle to 180ml, keep the temperature at 35±0.5°C and continue to rotate After culturing for 14 days, replace with fresh nutrient solution, and co-cultivate for 26-28 days;
[0029] ...
Embodiment 2
[0037] A. Human embryonic lung diploid cells KMB 17 For the 20th generation, use 0.03% trypsin + 0.02% ethylenediaminetetraacetic acid to disperse and suspend the monolayer cells in the culture medium at a speed of 8-12 rpm and a temperature of 37±0.5°C Culture for 3-6 days to make the cells grow into a dense monolayer;
[0038] B. KMB that will grow into a dense monolayer 17 Discard the nutrient solution for the cells, and after digestion, collect the cells with MEM medium containing 2% serum, and add live attenuated hepatitis A vaccine virus seeds to the cells according to the ratio of the number of viruses to the number of cells at 1:40-60 Stir for 2 hours at 37°C with a speed of 20 rpm to suspend and adsorb the virus, add maintenance solution containing 5% serum to the final volume of each spinner bottle to 180ml, keep the temperature at 35±0.5°C and continue to rotate After culturing for 14 days, replace with fresh nutrient solution, and co-cultivate for 26-28 days;
...
Embodiment 3
[0047] A. Human embryonic lung diploid cells KMB 17 For passage 40, use 0.05% trypsin + 0.04% ethylenediaminetetraacetic acid to disperse and suspend the monolayer cells in the culture medium at a speed of 8-12 rpm and a temperature of 37±0.5°C Culture for 3-6 days to make the cells grow into a dense monolayer;
[0048] B. KMB that will grow into a dense monolayer 17 Discard the nutrient solution for the cells, and after digestion, collect the cells with MEM medium containing 5% serum, and add live attenuated hepatitis A vaccine virus seeds to the cells according to the ratio of the number of viruses to the number of cells at 1:40-60 Stir for 0.5 hours at a temperature of 37°C and a speed of 120 rpm to suspend and adsorb the virus, add maintenance solution containing 10% serum to the final volume of each spinner bottle to 180ml, keep the temperature at 35±0.5°C and continue to rotate After culturing for 14 days, replace with fresh nutrient solution, and co-cultivate for 26-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com