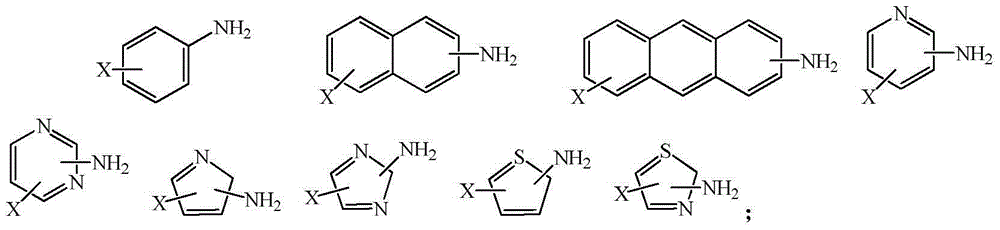
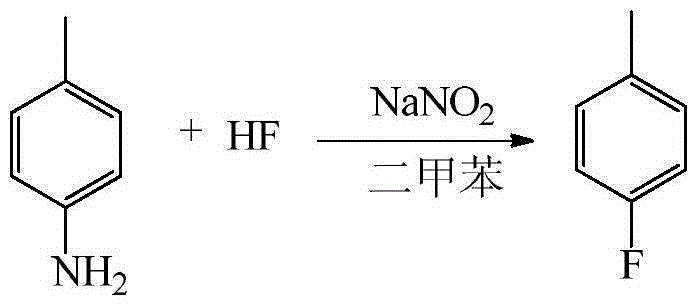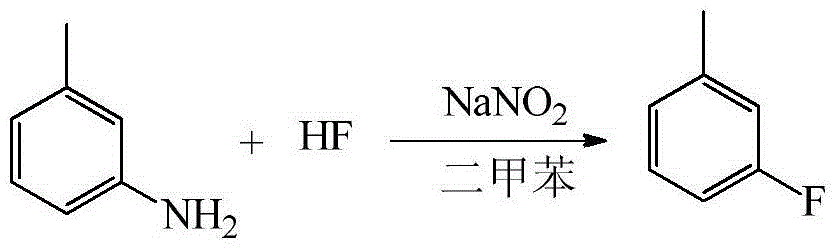Synthetic method of fluorine-containing aromatic compounds
A technology of aromatic compounds and aromatic compounds, applied in chemical instruments and methods, organic chemistry, preparation of halogenated hydrocarbons, etc., can solve problems such as inability to form precipitation, industrial production limitations, fast decomposition reaction speed, etc., to achieve decomposition and release heat Easy to control, enhance international market competitiveness, and controllable decomposition speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis reaction formula of p-fluorotoluene:
[0039]
[0040] Specifically:
[0041] (1) Dissolve 50g (0.466mol) of p-toluidine in 100g of xylene, freeze and cool the 500ml plastic reactor to -10°C, add 150g (7.5mol) of anhydrous hydrogen fluoride to the reactor and stir it under magnetic force, Slowly add the above-mentioned toluidine / xylene solution dropwise at ~-5°C for about 1 hour to form a salt.
[0042] (2) Slowly add 32 g (0.464 mol) of sodium nitrite solid to the reaction solution after salt formation at -10 to -5° C. to carry out diazotization reaction. During the diazotization process, the reaction is exothermic, and it is advisable to add the sodium nitrite so that the temperature of the feed liquid is not higher than 0°C. After the reaction was completed, the stirring was continued for 15 min, and the reaction solution was allowed to stand for 30 min.
[0043] (3) Transfer the diazo solution in the lower layer after standing still...
Embodiment 2
[0044] Embodiment 2: the synthesis of m-fluorotoluene
[0045]
[0046] Steps:
[0047] (1) Dissolve 50g (0.466mol) of m-toluidine in 100g of xylene, freeze and cool the 500ml plastic reactor to -10°C, add 150g (7.5mol) of anhydrous hydrogen fluoride to the reactor and stir it under magnetic force, Slowly add m-toluidine / xylene solution dropwise at ~-5°C for about 1 hour to form a salt.
[0048] (2) Slowly add 32 g (0.464 mol) of sodium nitrite solid to the reaction solution after salt formation at -10 to -5° C. to carry out diazotization reaction. Continue to stir for 15 minutes after the addition, and let the reaction solution stand for 40 minutes.
[0049] (3) Transfer the diazo solution in the lower layer after standing still to a frozen high-level tank for storage until use. Stir the remaining organic phase in the kettle and heat up to 40-45°C. After heating up, slowly drop the frozen diazonium solution into the organic phase to carry out the decomposition reaction, ...
Embodiment 3
[0050] Embodiment 3: the synthesis of o-fluorotoluene
[0051]
[0052] Steps:
[0053] (1) Dissolve 50 g (0.466 mol) o-toluidine in 100 g xylene. Freeze and cool down the 500ml plastic reactor to -10°C, add 150g (7.5mol) of anhydrous hydrogen fluoride to the reactor, and slowly add the o-toluidine / xylene solution dropwise at -10~-5°C for about 1 hour under magnetic stirring. A salt.
[0054] (2) Slowly add 32 g (0.464 mol) of sodium nitrite solid to the reaction solution after salt formation at -10 to -5° C. to carry out diazotization reaction. Continue to stir for 15 minutes after the addition, and let the reaction solution stand for 50 minutes.
[0055] (3) Transfer the diazonium liquid in the lower layer after standing still to a frozen high-level tank for storage, and stir the remaining organic phase in the kettle to raise the temperature to 40-45°C. After heating up, slowly drop the frozen diazonium solution into the organic phase to carry out the decomposition re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com