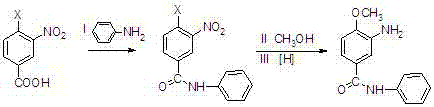
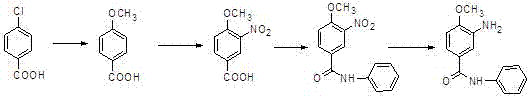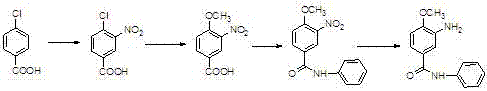A kind of preparation method of 3-amino-4-methoxybenzanilide
A methoxybenzalanilide and a technology for benzanilide, which is applied in the field of preparation of 3-amino-4-methoxybenzalanilide, can solve the problems of serious pollution, long steps, large three wastes and the like, and achieves the The effect of high product content, less three wastes, and avoiding environmental problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of preparation method of 3-amino-4-methoxybenzanilide, it comprises the steps:
[0034] (1) Preparation of 3-nitro-4-chlorobenzoanilide: In a 1000L reaction vessel, pump chlorobenzene (200kg), start stirring, and add 3-nitro-4-chlorobenzoic acid (50kg , percent), aniline (27kg), warm up to 70-80°C, add phosphorus trichloride (20kg) dropwise, after the dropwise addition, heat up to 100°C, keep warm for 2 hours, drop to room temperature, add water (400kg) dropwise , heating up to reflux, changing the reflux to distillation, steaming chlorobenzene at normal pressure, cooling, filtering, and drying to obtain 65.7kg of 3-nitro-4-chlorobenzanilide, which is a yellow-brown solid with a purity of 98.1%. The yield is 95.8%, and the melting point is 128-130°C;
[0035] (2) Preparation of 3-nitro-4-methoxybenzanilide: In a 1000L reaction vessel, draw methanol (400kg), start stirring, add 3-nitro-4-chlorobenzanilide ( 55.4kg), sodium methoxide (11.9kg), start stirring, hea...
Embodiment 2
[0038] This example provides a kind of synthetic method of 3-amino-4-methoxybenzanilide, and it comprises the steps:
[0039] (1) Preparation of 3-nitro-4-chlorobenzanilide: In a 1000L reaction vessel, pump chlorobenzene (200kg) and aniline (27kg), start stirring, and add 3-nitro-4-chlorobenzene Formic acid (50kg, 100%), heat up to 70-80°C, add thionyl chloride (40kg) dropwise, after the dropwise addition, heat up to 100°C, keep warm for 2 hours, drop to room temperature, add water (400kg) dropwise, heat up To reflux, change the reflux to distillation, steam chlorobenzene at normal pressure, cool down, filter, and dry to obtain 66.5kg of 3-nitro-4-chlorobenzanilide, which is a yellow-brown solid with a purity of 98.5%. It is 97%, and its melting point is 128-130°C;
[0040](2) Preparation of 3-nitro-4-methoxybenzanilide: In a 1000mL reaction vessel, draw methanol (400kg), start stirring, add 3-nitro-4-chlorobenzanilide ( 55.4kg), potassium hydroxide (12.3kg), heated to reflu...
Embodiment 3
[0043] This example provides a kind of synthetic method of 3-amino-4-methoxybenzanilide, and it comprises the steps:
[0044] (1) Preparation of 3-nitro-4-chlorobenzanilide: In a 1000L reaction vessel, add 3-nitro-4-chlorobenzoic acid (50kg, percent), dichlorobenzene (300kg), aniline (30kg), triphenyl phosphite (5kg), heat up to 115°C to reflux, toluene brings out water during reflux, after reflux for 6-7 hours, cool down, filter, and dry to obtain 3-nitro-4- 60.5kg of chlorobenzanilide is a yellow-brown solid with a purity of 98.0%, a yield of 88.2%, and a melting point of 128-130°C. Toluene mother liquor (containing catalyst) is recovered and applied mechanically;
[0045] (2) Preparation of 3-nitro-4-methoxybenzanilide: In a 1000L reaction flask, draw methanol (400kg), start stirring, add 3-nitro-4-chlorobenzanilide ( 55.4kg), sodium hydroxide (8.8kg), heated and refluxed for 8 hours, cooled, filtered, washed with water, and dried to obtain 51.9kg of 3-nitro-4-methoxybenz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com