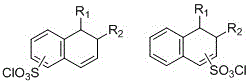
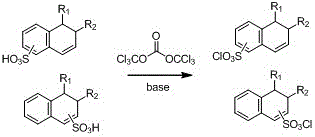
Method for synthesizing naphthoquinone sulfonyl chloride
A technology of naphthoquinone sulfonyl chloride and naphthoquinone sulfonic acid, which is applied in the fields of sulfonic acid preparation and organic chemistry, can solve the problems of large amount of diphosgene and solid phosgene and low reaction temperature, so as to ensure the safe operation and reaction of equipment The effect of mild temperature and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment is the synthesis of 2-diazo-1-naphthoquinone-4-sulfonyl chloride:
[0031] Add 25.02g (0.1mol) of 2-diazo-1-naphthoquinone-4-sulfonic acid and 600mL of 1,2-dichloroethane into a 1000mL reaction flask, and add 19.6g of bis(trichloromethyl)carbonate (0.067 mol). Under the condition of ice-water bath, 10.1 g (0.1 mol) of triethylamine was added dropwise to the reaction flask, and a white solid was produced in the reaction mixture at this time. After dropping, stir the reaction for 30min; slowly warm the reaction mixture to 40oC and stir the reaction for 2h. Cooled down to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was added to 500 mL of ice-water mixture, stirred for 5 min, and a solid precipitated out. Filter and wash the filter cake with a small amount of ice-water mixture. The solid product was dried under vacuum. 23.0 g of the product was obtained with a yield of 81%. HPLC detection purity: 95.7%. ...
Embodiment 2
[0033] This embodiment is the synthesis of 2-diazo-1-naphthoquinone-5-sulfonyl chloride:
[0034] Add 25.02g (0.1mol) of 2-diazo-1-naphthoquinone-5-sulfonic acid and 300mL of 1,2-dichloroethane into a 1000mL reaction bottle, add 50.5g (0.5mol) of triethylamine in an ice-water bath Under the conditions, a solution prepared by 29.2 g (0.1 mol) of bis(trichloromethyl)carbonate and 300 mL of 1,2-dichloroethane was added dropwise into the reaction flask. At this point a white solid formed in the reaction mixture. After dropping, stir and react for 30 minutes; keep stirring and react for 4.0 hours. The reaction mixture was concentrated under reduced pressure. The residue was added to 500 mL of ice-water mixture, stirred for 5 min, and a solid precipitated out. Filter and wash the filter cake with a small amount of ice-water mixture. The solid product was dried under vacuum. 24.2 g of the product was obtained with a yield of 85.0%.
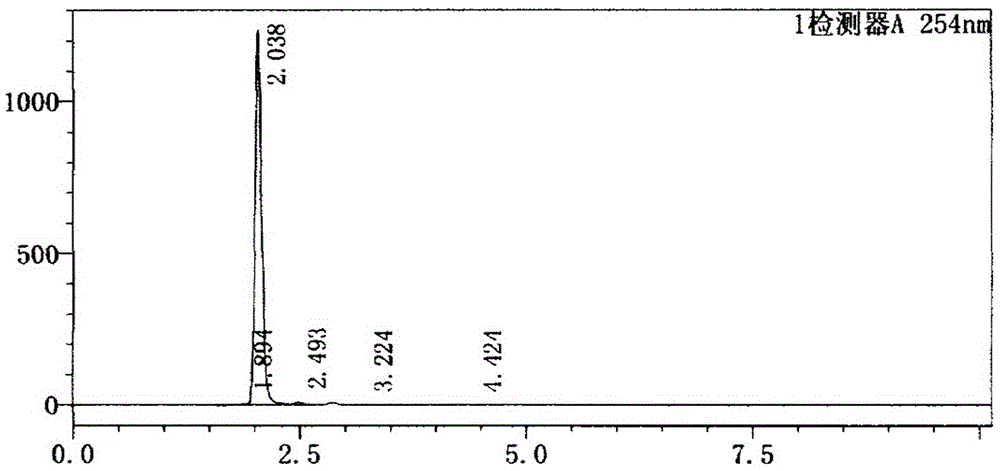
[0035] figure 1 HPLC analysis spectrum of n...
Embodiment 3
[0037] This embodiment is the synthesis of 2-diazo-1-naphthoquinone-5-sulfonyl chloride:
[0038]Add 25.02g (0.1mol) of 2-diazo-1-naphthoquinone-5-sulfonic acid and 600mL of 1,2-dichloroethane into a 1000mL reaction flask, and add 19.6g of bis(trichloromethyl)carbonate (0.067 mol). Under the condition of ice-water bath, 10.1 g (0.1 mol) of triethylamine was added dropwise to the reaction flask, and a white solid was produced in the reaction mixture at this time. After dropping, stir and react for 30min; slowly warm the reaction mixture to 40oC and stir for 2.0h. Cooled down to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was added to 500mL of ice-water mixture, stirred for 5min, and a solid precipitated out. Filter and wash the filter cake with a small amount of ice-water mixture. The solid product was dried under vacuum. 24.2 g of the product was obtained with a yield of 85%. HPLC detection purity: 97.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com