Monoclonal antibodies resistant to PLAG (platelet-aggregation-stimulating) domain of human PDPN (Podoplanin) and application of monoclonal antibodies
A technology of monoclonal antibody and deposit number, which is applied in the field of monoclonal antibody and can solve problems such as the lack of effective means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
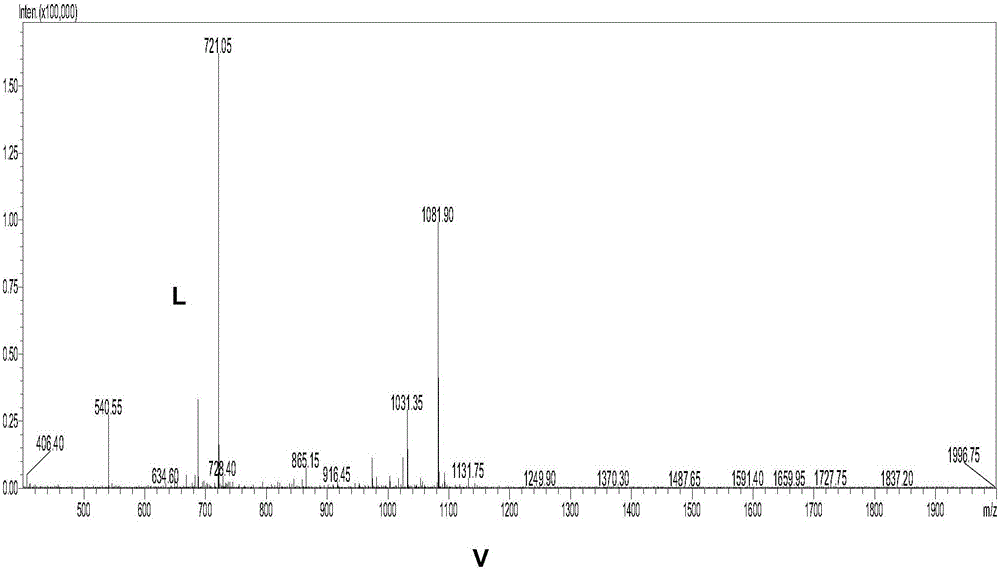
[0040] Example 1: Immunogen preparation—a 22-amino acid polypeptide fragment (DTETTGLEGGVAMPGAEDDVVC) of the PLAG region of artificially synthesized human PDPN (see mass spectrogram figure 1 ) was coupled with keyhole limpet hemocyanin (KLH) to form DTETTGLEGGVAMPGAEDDVVC-KLH as an immunogen, and Balb / c mice were immunized. (Completed by Shanghai Ziyu Biotechnology Co., Ltd.)
Embodiment 2
[0041] Embodiment 2: the preparation of the monoclonal antibody of the PLAG region of specific anti-PDPN
[0042] We prepared the monoclonal antibody of the present invention using conventional immunological methods and hybridoma technology (Köhler and Milstein, Nature, 215:495-497, 1975).
[0043] First, 8-week-old female Balb / c mice (Experimental Animal Center, Shanghai Academy of Sciences) were immunized with artificially synthesized conjugated 22-peptide-KLH (DTETTGLEGGVAMPGAEDDVVC-KLH) for three times with an interval of four weeks. The first two times were multi-point subcutaneous injection on the back plus intraperitoneal injection, and the third time was injection through the mouse tail vein plus intraperitoneal injection.
[0044] After the serum antibody titer of the immunized mice reached a high enough level, the animals were sacrificed and spleen cells were isolated. Using standard monoclonal antibody cell fusion technology (Kǒhler and Milstein, Nature, 215: 495-4...
Embodiment 3
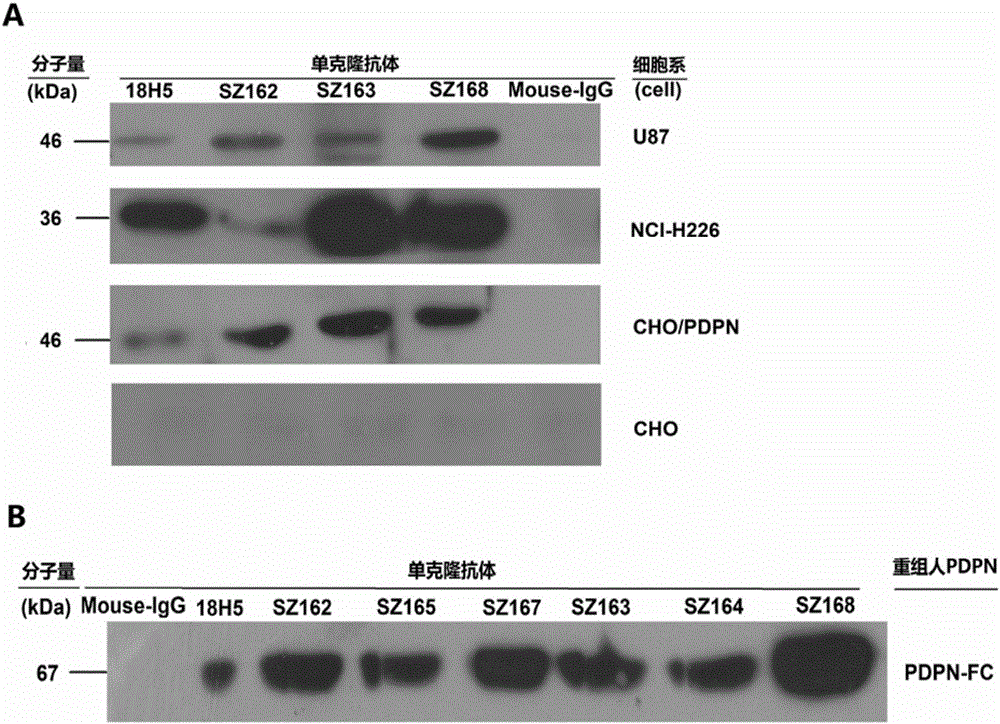
[0047] Example 3: Biochemical properties of monoclonal antibodies of the present invention
[0048] (1) It was confirmed by the double-diffusion method that the monoclonal antibodies SZ-163 and SZ-168 of the present invention belong to the IgG1 subclass.
[0049] (2) Inoculate the clones producing monoclonal antibodies SZ-163 and SZ-168 in the peritoneal cavity of Balb / c mice to produce ascites, and use protein G-Sepharose-4B ( Pharmacia Product) column purification, every 1mL of ascites can be purified to obtain 1.0mg of IgG.
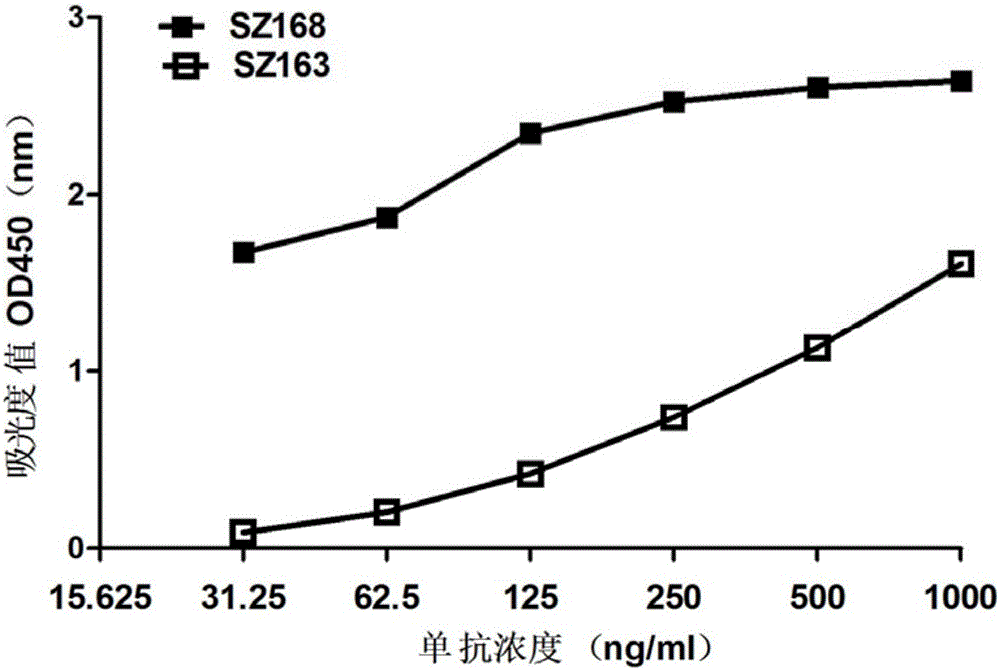
[0050] (3) Determination of the specificity of SZ-163 and SZ-168 by ELISA experiment: the artificially synthesized PDPN 22 peptide (1 μg / ml) was coated overnight at 4°C, washed with PBS-0.05%-Tween 20, and washed with 2% BSA-PBS blocked overnight. Dilute SZ-163 and SZ-168 from 1 μg / ml to 6 concentrations (1 μg / ml, 500ng / ml, 250ng / ml, 125ng / ml, 62.5ng / ml, 31.25ng / ml), 37°C React for 1.5 hours. After washing, horseradish peroxidase-labeled goat anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com