Preparation method for hydrogenation catalyst for polycyclic aromatic hydrocarbons, catalyst and application thereof
A technology for hydrogenation catalysts and polycyclic aromatic hydrocarbons, applied in chemical instruments and methods, physical/chemical process catalysts, molybdenum sulfide, etc. High selectivity and other issues, to achieve the effect of high hydrodesulfurization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
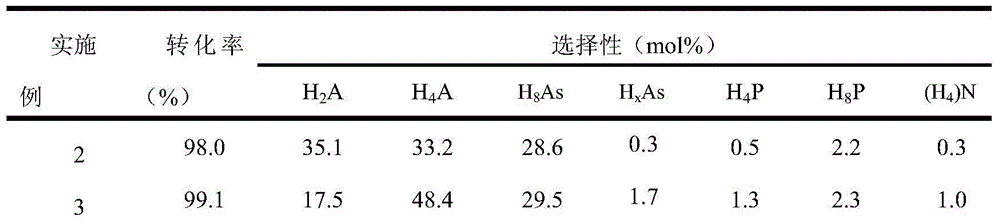
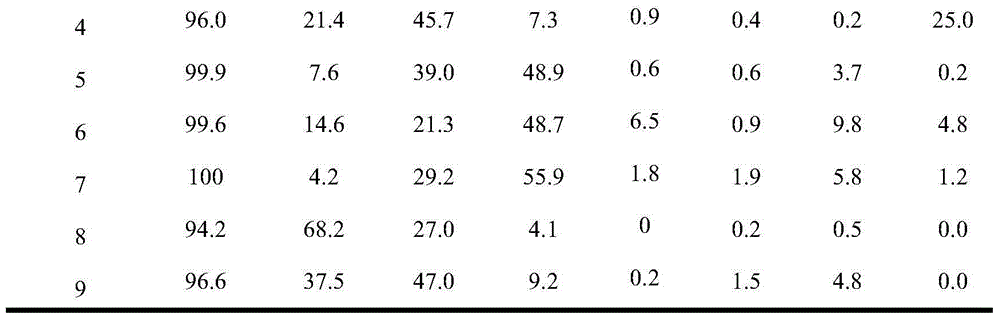
Examples
Embodiment 1
[0025] Catalyst preparation:
[0026] Prepare aqueous solution A containing ammonium tetrathiomolybdate (ATTM), the concentration of ATTM is 0.15mol / L. Prepare aqueous solution B containing hydrazine hydrate, the concentration of hydrazine hydrate is 16mol / L. A mixture C containing nonionic surfactant, n-hexanol and cyclohexane was prepared, wherein the nonionic surfactant was 10.6 g, n-hexanol was 8.0 g and cyclohexane was 13.3 g. Add 4ml of ATTM aqueous solution A to mixture C under stirring, and stir for 30 minutes to obtain stable microemulsion D. Add 1 ml of hydrazine hydrate aqueous solution B to the above microemulsion D, and stir for 30 minutes to obtain stable microemulsion E. The microemulsion E was transferred into an autoclave, sealed and left to crystallize at 185°C for 24 hours. Cool down to room temperature naturally after taking it out. After filtering, washing with deionized water and absolute ethanol three times, and vacuum drying at 70 °C for 12 h, the b...
Embodiment 2
[0029] Add 0.075g of the molybdenum disulfide catalyst prepared in Example 1 (5% by weight based on anthracene) into the 100mL autoclave reactor of the slurry bed reaction system, and then add 1.5g of anthracene and 28.5g of n-hexadecane. After loading the kettle, replace the air with hydrogen 3 times (close the exhaust valve first, then open the intake valve each time, pressurize with 100ml / min hydrogen to 2MPa, then close the intake valve, and then open the exhaust valve to empty.), boost the pressure to 4MPa, then raise the temperature to 325°C, and carry out the hydrogenation reaction at a stirring speed of 300r / min for 4 hours.
Embodiment 3
[0031] Add 0.075g of the molybdenum disulfide catalyst prepared in Example 1 (5% by weight based on anthracene) into the 100mL autoclave reactor of the slurry bed reaction system, and then add 1.5g of anthracene and 28.5g of n-hexadecane. After filling the kettle, replace the air with hydrogen for 3 times (the replacement operation is the same as in Example 2), increase the pressure to 4MPa, and then raise the temperature to 350°C, and carry out the hydrogenation reaction for 4 hours at a stirring speed of 300r / min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com