Ternary brush polymer with amphipathy and pH value responsiveness, and nanoporous capsules
A nanoporous vesicle and responsive technology, applied in the field of self-assembled polymer materials, can solve problems such as difficult removal of copper ions, achieve the effect of improving reaction rate and efficiency, and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
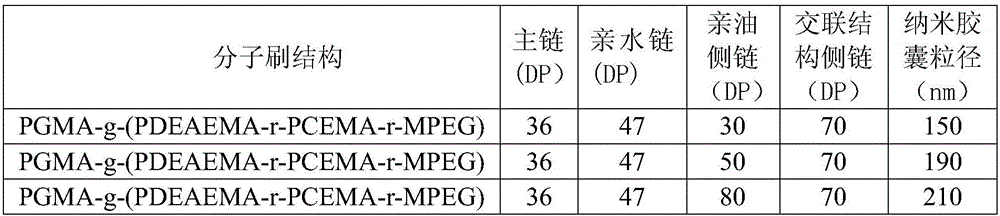
[0034] Example 1: Preparation of amphiphilic pH-responsive ternary molecular brushes by mercapto-epoxy click chemistry
[0035] (1) Synthesis of PGMA main chain
[0036] Take 1 part of ethyl 2-bromoisobutyrate initiator, 200 parts of glycidyl methacrylate (GMA), 100 parts of diphenyl ether, 1 part of CuBr and 1 part of N,N,N',N',N" - Pentamethyldiethylenetriamine (PMDETA), carry out ATRP reaction at room temperature under the protection of nitrogen, and obtain polyglycidyl methacrylate (PGMA) with a degree of polymerization (DP) of 120 after reaction for 1 h.
[0037] (2) Synthesis of side chains
[0038] Synthesis of hydrophilic side chains: 100 parts of monomethoxypolyethylene glycol (Mn=5000) and 200 parts of thionyl chloride were reacted for 24 hours under nitrogen protection. After the reaction was completed, distilled under reduced pressure to obtain chloromonomethoxypolyethylene glycol. Take 20 parts of monochloromonomethoxypolyethylene glycol, 120 parts of thiourea,...
Embodiment 2
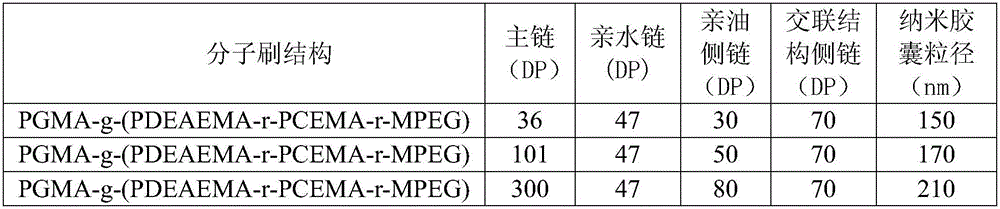
[0045] Embodiment 2: Preparation of amphiphilic pH value responsive ternary molecular brush by mercapto-epoxy click chemistry
[0046] (1) Synthesis of PGA main chain
[0047] Take 1 part of ethyl 2-bromoisobutyrate initiator, 200 parts of glycidyl acrylate (GA), 100 parts of diphenyl ether, 1 part of CuBr and 1 part of N, N, N', N', N"-5 Methyldiethylenetriamine (PMDETA) was subjected to ATRP reaction at room temperature under the protection of nitrogen, and polyglycidyl methacrylate (PGA) with a degree of polymerization (DP) of 120 was obtained after reaction for 1 h.
[0048] (2) Synthesis of side chains
[0049] Synthesis of hydrophilic side chains: 100 parts of monomethoxypolyethylene glycol (Mn=5000) and 200 parts of thionyl chloride were reacted for 24 hours under nitrogen protection. After the reaction was completed, distilled under reduced pressure to obtain chloromonomethoxypolyethylene glycol. Take 20 parts of monochloromonomethoxypolyethylene glycol, 120 parts o...
example 3
[0056] Example 3: Preparation of amphiphilic pH-responsive ternary molecular brushes by mercapto-epoxy click chemistry
[0057] (1) Synthesis of PGMA main chain
[0058] Take 1 part of ethyl 2-bromoisobutyrate initiator, 200 parts of glycidyl methacrylate (GMA), 100 parts of diphenyl ether, 1 part of CuBr and 1 part of N,N,N',N',N" -Pentamethyldiethylenetriamine (PMDETA), carry out ATRP reaction at room temperature under the protection of nitrogen, and obtain polyglycidyl methacrylate (PGMA) with a degree of polymerization (DP) of 120 after reacting for 7 hours.
[0059] (2) Synthesis of side chains
[0060] Synthesis of hydrophilic side chains: 100 parts of monomethoxypolyethylene glycol (Mn=5000) and 200 parts of thionyl chloride were reacted for 24 hours under nitrogen protection. After the reaction was completed, distilled under reduced pressure to obtain chloromonomethoxypolyethylene glycol. Take 20 parts of monochloromonomethoxypolyethylene glycol, 120 parts of thiour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com