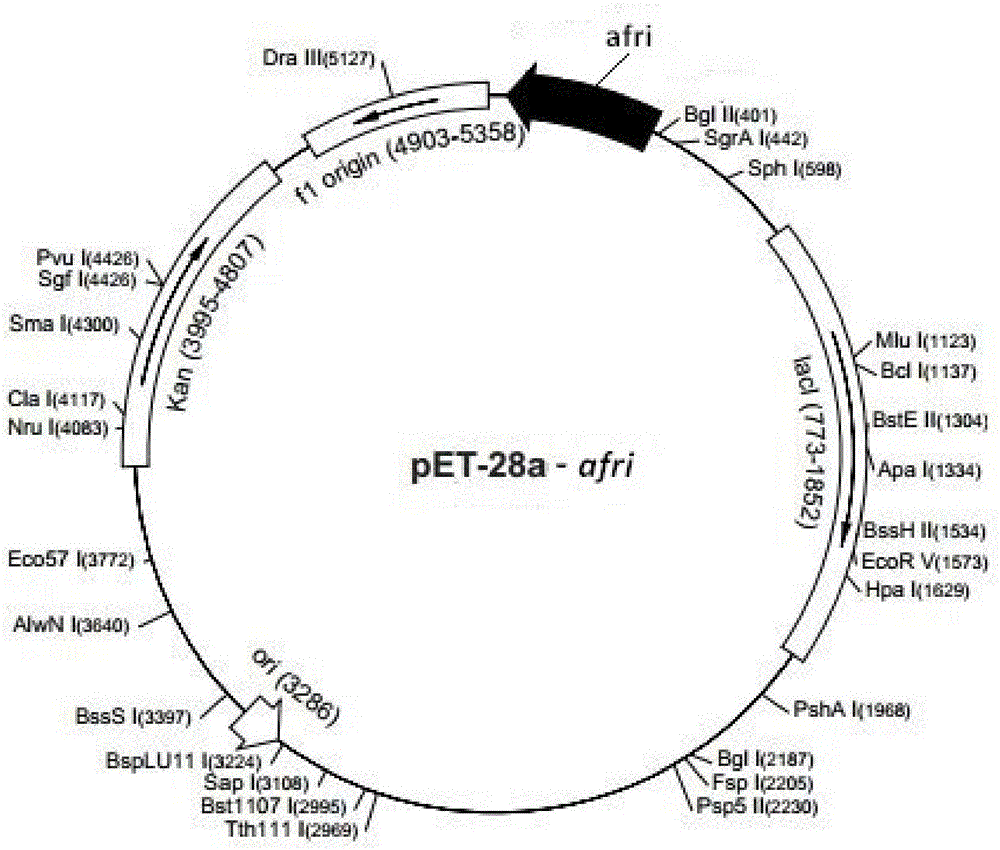
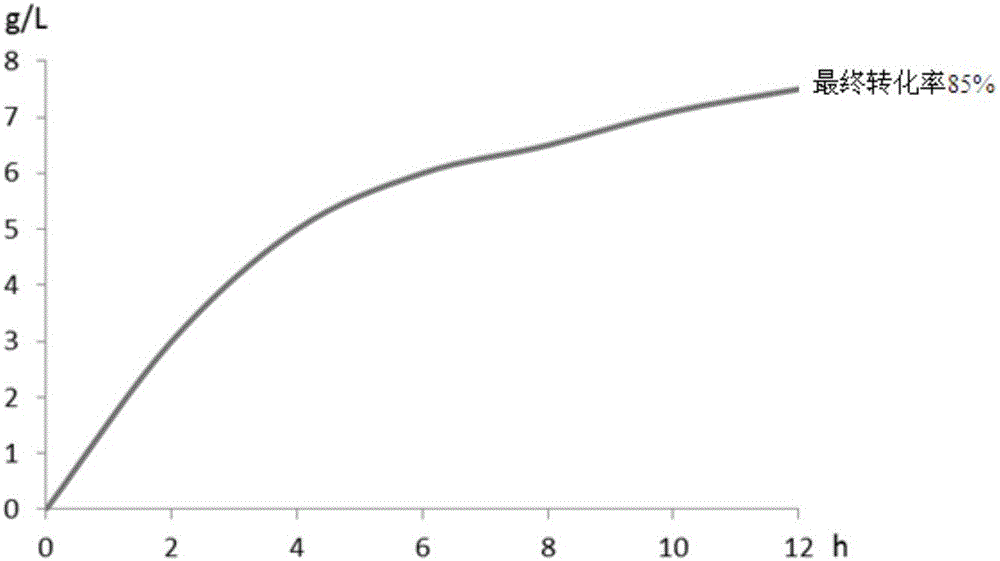
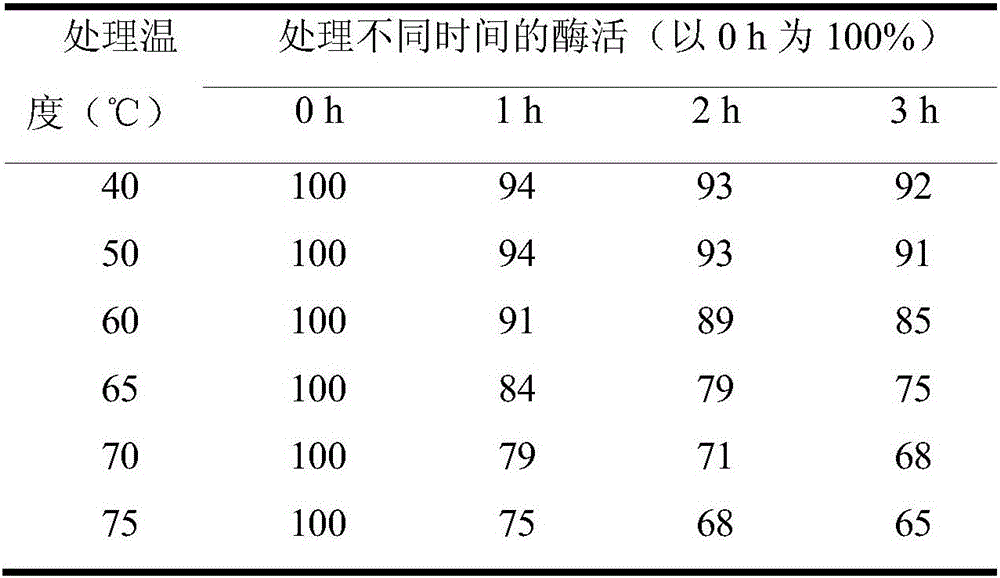
L-ribose isomerase and application thereof for biologically preparing L-ribose
An isomerase and ribose technology, which is applied to L-ribose isomerase and its application field in biological preparation of L-ribose, can solve the problem of low catalytic efficiency of L-ribulose, and achieves wide application prospects and Economic value, good stability, high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Genome extraction from fermentative Actinoleafermentans NX-1.
[0040] Actinoleafermentans NX-1 was preserved in our laboratory. Genomic DNA of actinoleafermentans NX-1 in the logarithmic growth phase was extracted with GenomicDNA Purification Kit (Takara, Dalian), and analyzed by agarose gel. The obtained bacterial genomes were detected by electrophoresis.
Embodiment 2
[0041] Example 2: Cloning of L-ribose isomerase coding gene (afri) and construction of recombinant bacteria.
[0042] 2.1 PCR amplification of afri gene.
[0043] According to the existing functionally unknown sequence on GeneBank (Genbank Accession No. WP_034244055), use VectorNTI software to design primers Primer1 and Primer2, the primer sequence is:
[0044] Primer 1: 5'-CG GGATCC( BamHI ) ATGACCCGTACGTATGTGACCCGTC-3';
[0045] Primer 2: 5'-CCC AAGCTT( Hind III )TTAGCGAATGTGCGTCACCAGACGG-3';
[0046] Using the genome obtained in Example 1 as a template, the gene fragment of Actinocytocellum fermentum was amplified.
[0047] The PCR amplification system is: 2 μL of genomic DNA, 1 μL of each of primers Primer1 and Primer2, 2 μL of dNTP, 2.5 μL of 10×Tag buffer, 0.5 μL of Ex-Tag polymerase, ddH 2 O14 μL.
[0048] The PCR reaction program was as follows: pre-denaturation at 94°C for 5 minutes, denaturation at 94°C for 30 seconds; then annealing at 54°C for 2 minutes, e...
Embodiment 3
[0066] Example 3: Induced expression of L-ribose isomerase.
[0067] The recombinant E. coli BL21-AFRI was inoculated in 5 mL of LB liquid medium supplemented with 25 μg / mL kanamycin, and cultured on a shaker at 37°C overnight; then transferred to 100 mL of LB medium (containing 25μg / mL kanamycin) in a 500mL shake flask, cultured on a shaker at 37°C for 2-3h, until OD 600 At about 0.6, add IPTG for induction (IPTG final concentration 1mmol / L), or add 1g / L lactose for induction, and then continue to induce expression for 6h, and collect the bacteria by centrifugation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com