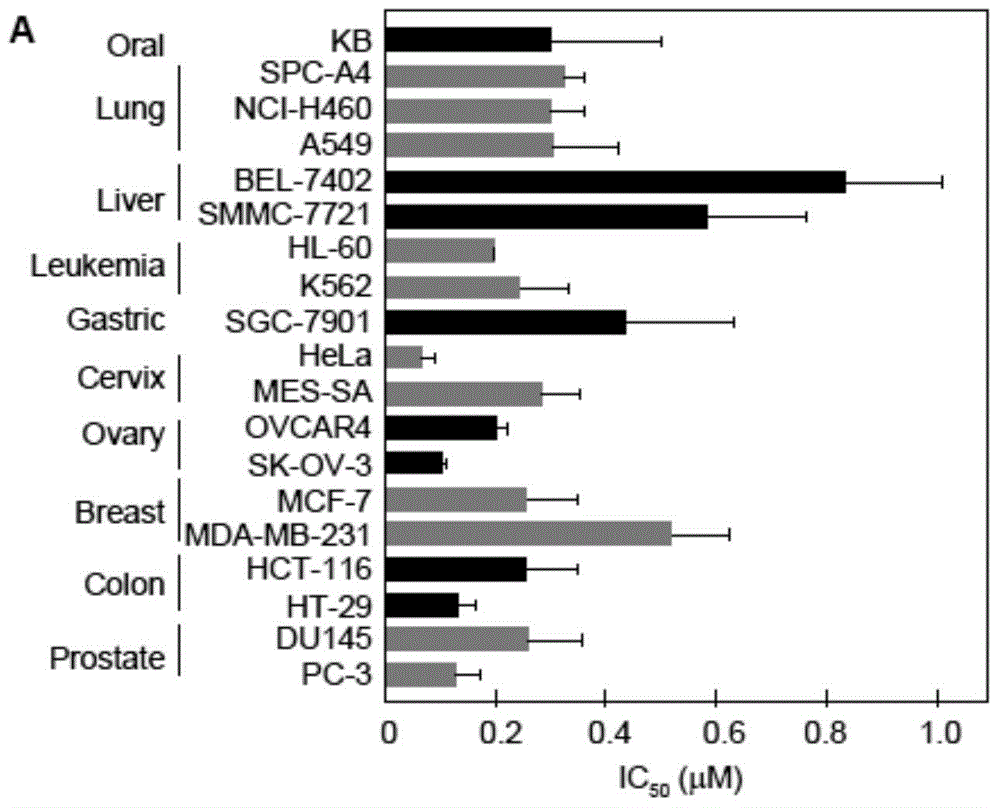
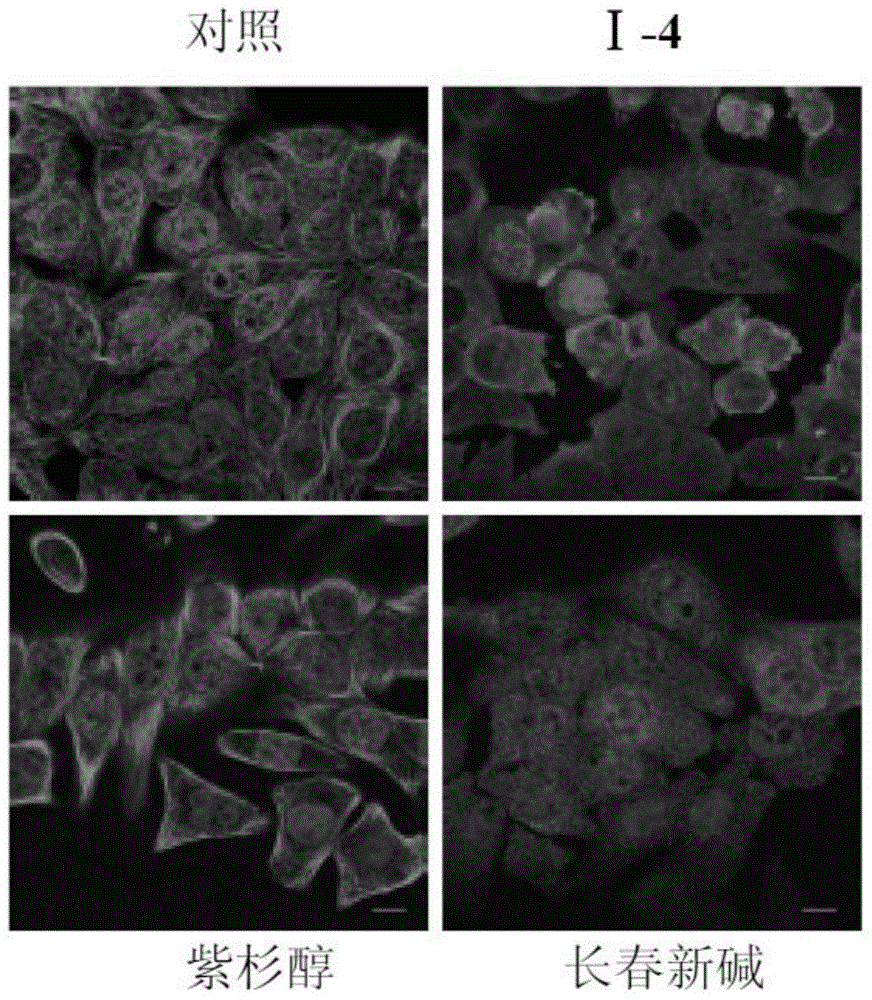
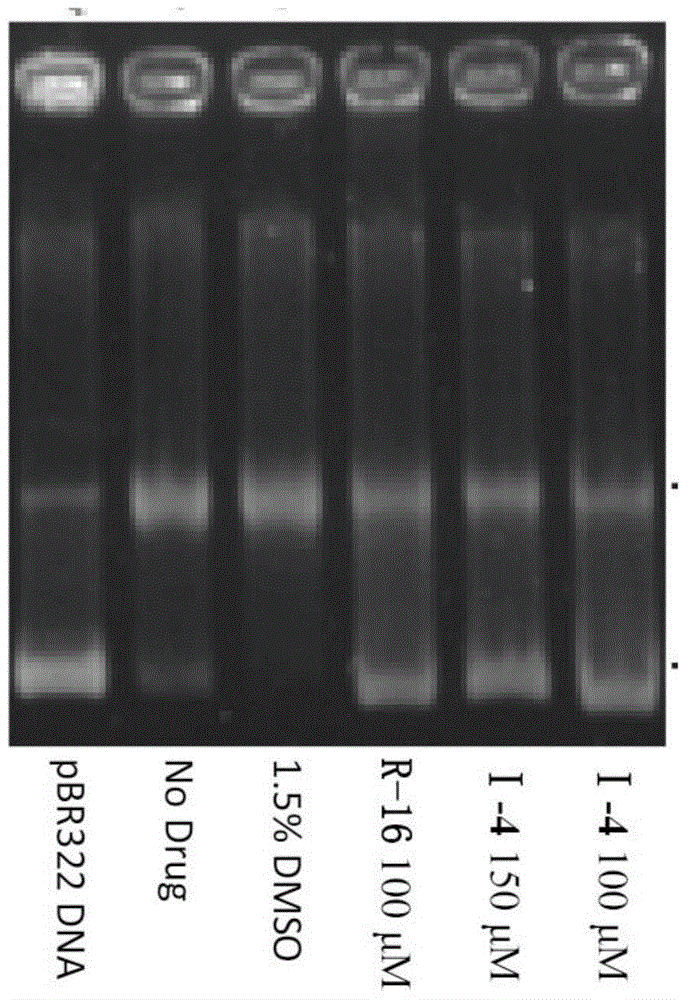
Alpha-carboline compounds, preparation method thereof and purpose thereof
A compound, unsubstituted technology, applied to the core compound containing α-carboline, the application field in medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0153] The preparation of formula I compound
[0154] Another object of the present invention is to provide the preparation method of the compound shown in general formula I, and synthetic route is as shown in Scheme 1 or 2:
[0155] plan 1
[0156]
[0157] a) Compound 1 (3-aldehydochromone) and compound 2 (o-nitrophenylacetonitrile) are condensed to generate compound 3 under certain conditions, and the conditions are acetic anhydride / sodium acetate system, or titanium tetrachloride / pyridine system;
[0158] b) Compound 3 is heated and ring-closed under the action of a reducing agent (hydrogen, iron powder, zinc powder, sulfide) to generate compound 4 / I.
[0159] c) Compound 4 is substituted under the action of base (sodium hydride, potassium tert-butoxide, sodium tert-butoxide) to further generate compound I.
[0160] Preferably, the conditions of step a) are: compound 1 and compound 2 are dissolved in acetic anhydride, stirred overnight at 70-100° C. for reaction unde...
Embodiment 1
[0203] Example 1 Preparation of (E / Z)-2-(2-nitrobenzene)-3-(4-oxo-4H-chromone-3-substituted) acrylonitrile (compound 3a)
[0204]
[0205] In a 25mL round bottom flask, add 100mg (0.574mmol) of compound 3-aldehyde-chromone 1a, 93mg (0.574mmol) of o-nitrophenylacetonitrile, 47mg (0.574mmol) of sodium acetate, 5mL of acetic anhydride, and place in an oil bath at 90°C Stir and heat in the pot overnight. The next day, the temperature was cooled to room temperature, and the reaction was quenched by pouring 150 mL of ice water. 50mL DCM was extracted three times, the combined organic phase was washed with 50mL saturated brine, then dried over anhydrous sodium sulfate, the solvent was evaporated to dryness by a rotary evaporator, and the crude product was purified by silica gel column chromatography (petroleum ether / ethyl acetate=4:1) to obtain light yellow The solid is 162mg, and the yield is 88%.
[0206] 1 HNMR (300MHz, Chloroform-d) δ9.03(s, 1H), 8.27(d, J=9.4Hz, 1H), 8.18(...
Embodiment 2
[0213] Example 2 Preparation of (E / Z)-2-(2-nitrobenzene)-3-(6-methyl-4-oxo-4H-chromone-3-substituted) acrylonitrile (compound 3b)
[0214]
[0215] 95 mg (0.5 mmol) of 3-formyl 6-methylchromone 1b was used as the raw material, referring to the synthesis process of compound 3a, 146 mg of light yellow solid was obtained, with a yield of 88%.
[0216] 1 HNMR (300MHz, Chloroform-d) δ9.01(s, 1H), 8.18(d, J=8.2Hz, 1H), 8.04(s, 1H), 7.79–7.71(m, 1H), 7.69–7.52(m ,4H),7.46(d,J=8.6Hz,1H),2.49(s,3H).
[0217] LC-MS: 333 (M+1).
[0218] Preparation of (2-hydroxy-5-methylphenyl)(9H-pyrido[2,3-b]indole-3-substituted)methanone (compound Ⅰ-2)
[0219]
[0220] 100mg (0.3mmol) of 3b was used as the starting material, referring to the synthesis process of compound Ⅰ-1, 52mg of yellow solid was obtained, with a yield of 58%.
[0221] 1 HNMR (300MHz, DMSO-d 6 )δ13.62(s,1H),12.11(s,1H),8.68(d,J=7.7Hz,1H),8.19(d,J=8.7Hz,1H),8.05–7.95(m,1H), 7.91(s,1H),7.57–7.42(m,2H),7.33–7.21(m,1H),7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com