Vinylene carbonate-based lithium ion battery polymer electrolyte and preparation method as well as application thereof
A polyvinylene carbonate-based lithium and polyvinylene carbonate-based technology is applied in the application field of room temperature all-solid-state lithium-ion batteries, and can solve the problems of low discharge specific capacity, low ionic conductivity, low mechanical strength, etc. High ionic conductivity, easy availability of materials, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
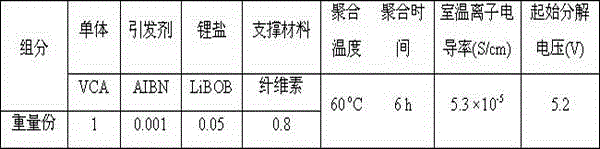
[0041] The LiBOB / VCA solution was prepared in a glove box filled with argon, and after adding AIBN to the LiBOB / VCA solution, the solution was injected into a lithium battery with cellulose as a support material. The battery is placed in 60 o After heating at C for 6 hours, VCA polymerized in situ inside the battery to obtain a polymer, and at the same time compounded with support materials and lithium salts to obtain a polymer electrolyte for lithium-ion batteries.
[0042] The mass fraction of AIBN in the LiBOB / VCA solution was 0.1%.
[0043] The ratio of raw materials used to prepare polymer electrolytes for lithium-ion batteries is shown in Table 1. The ion conductivity of polymer electrolytes for lithium-ion batteries prepared at room temperature is 5.3×10 -5 S / cm, the initial decomposition voltage is 5.2V.
[0044] Table 1:
[0045]
Embodiment 2
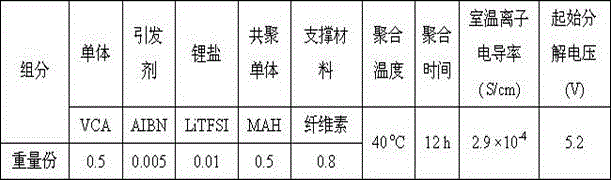
[0047] MAH was added to VCA in an argon-filled glove box to prepare a LiTFSI / VCA-MAH solution. After adding AIBN to the LiTFSI / VCA-MAH solution, the solution was injected into a lithium battery with cellulose as a support material. The battery is placed in 40 o After heating at C for 12 hours, VCA-MAH polymerized in situ inside the battery to obtain a copolymer, and at the same time compounded with support materials and lithium salts to obtain a lithium-ion battery polymer electrolyte.
[0048] The mass ratio of VCA and MAH was 5:5, and the mass fraction of AIBN in LiTFSI / VCA-MAH solution was 0.5%.
[0049] The ratio of raw materials used to prepare polymer electrolytes for lithium-ion batteries is shown in Table 2. The ion conductivity of polymer electrolytes for lithium-ion batteries prepared at room temperature is 2.9 × 10 -4 S / cm, the initial decomposition voltage is 5.2V.
[0050] Table 2:
[0051]
Embodiment 3
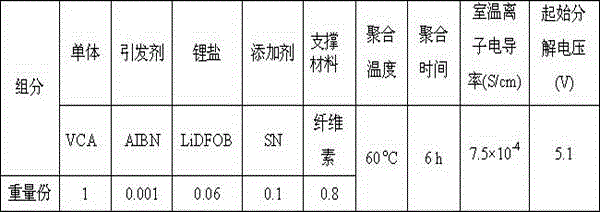
[0053] Preparation of LiClO by adding VAc to VCA in an argon-filled glove box 4 / VCA-VAc solution, to LiClO 4 After adding AIBN to the VCA-VAc solution, the solution was injected into a lithium battery supported by glass fiber. The battery is placed in 60 o After heating at C for 8 hours, VCA-VAc polymerized in situ inside the battery to obtain a copolymer, and at the same time compounded with support materials and lithium salts to obtain a lithium-ion battery polymer electrolyte.
[0054] Wherein the mass ratio of VCA and MAH is 8:2, AIBN is in LiClO 4 The mass fraction in VCA-VAc solution is 0.8%.
[0055] The ratio of raw materials used to prepare polymer electrolytes for lithium-ion batteries is shown in Table 3. The ion conductivity of polymer electrolytes for lithium-ion batteries prepared at room temperature is 1.7×10 -4 S / cm, the initial decomposition voltage is 4.6V.
[0056] table 3:
[0057]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com