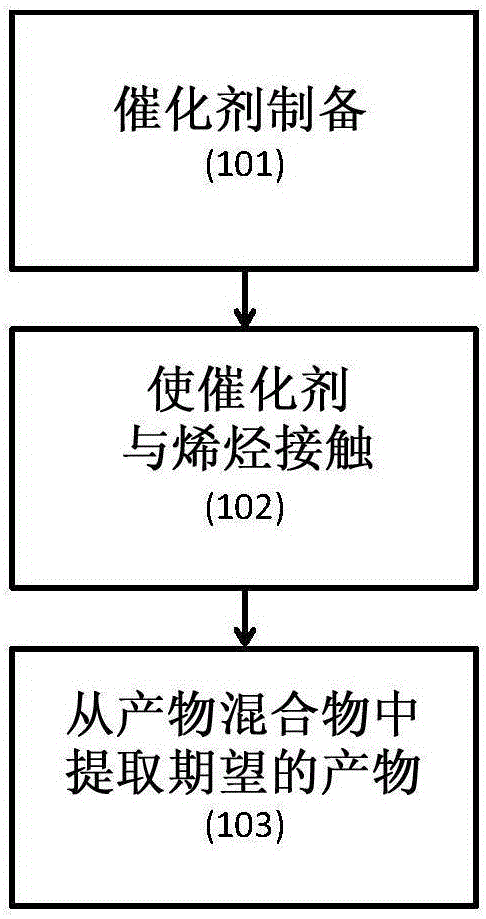
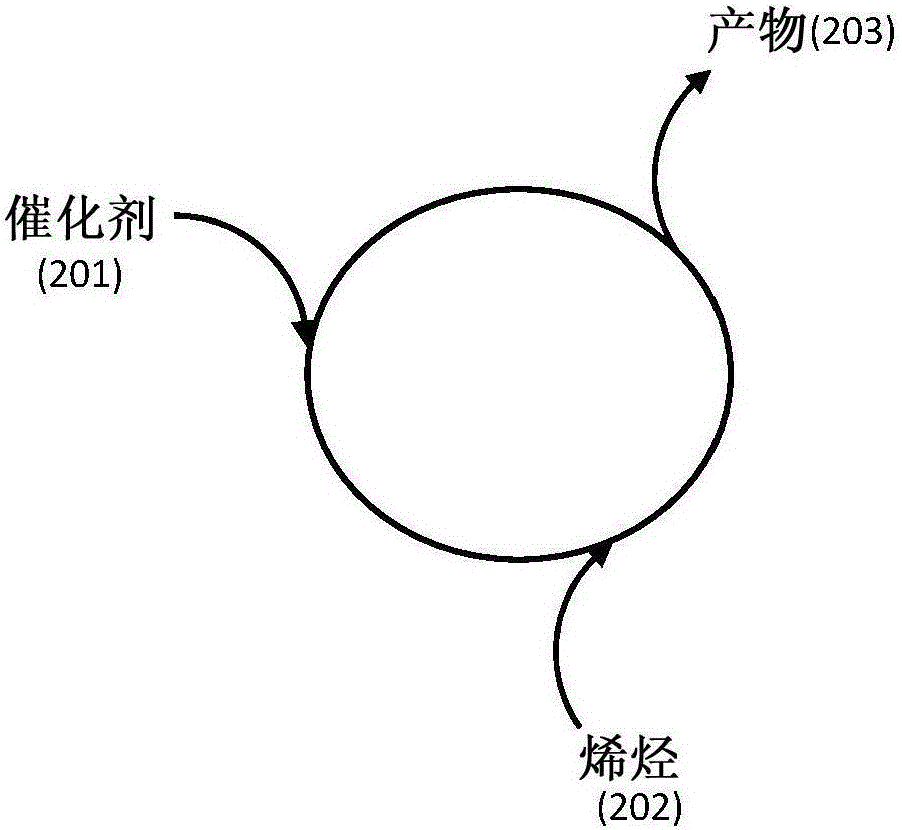
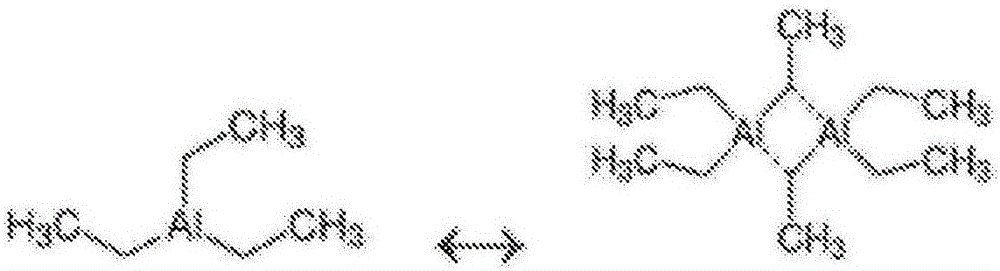Catalyst compositions for selective dimerization of ethylene
A technology of catalyst and composition, which is applied in the field of catalyst system of downstream products, and can solve the problem of too long initial induction period of polymer formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1 shows a catalyst system comprising a tetrasubstituted titanate, dibutyl ether and trialkylaluminum, and its use in the production of α-olefins from olefins, in particular 1-butene from ethylene use in . The results are summarized in Table 1.
[0106] Example 1a. The reaction was carried out in a batch reactor (Parr 300ml autoclave model 4566 Mini Bench Reactor) at 60°C and 23 bar. This temperature and pressure is maintained in the reactor throughout the reaction. 0.25 ml of tetra-n-butyl titanate (DorfKETAL) and 0.25 ml of dibutyl ether (Aldrich) were introduced into 50 ml of n-hexane (Aldrich). 1.8 ml of a 1M triethylaluminum solution in n-hexane was added thereto. The catalyst system in hexane was introduced into the reactor. The reaction system was heated to 60° C. with stirring and pressurized to 23 bar with ethylene for 1 hour. After depressurization, the product was collected in an adjacent vessel.
[0107] Example 1b (comparative). Example 1a was ...
Embodiment 2
[0112] Example 2 shows a catalyst system comprising a tetraalkyl titanate, a silicate and a trialkyl group, and its use in a process for the production of α-olefins from olefins, in particular 1-butene from ethylene. use. The results are summarized in Table 2.
[0113] Example 2a. The reaction was carried out in a batch reactor (Parr 300 ml autoclave model 4566 Mini Bench Reactor) at 60°C and 23 bar. This temperature and pressure is maintained in the reactor throughout the reaction. 0.25 ml of tetra-n-butyl titanate (DorfKETAL) and 0.25 ml of tetraethyl silicate (Aldrich) were introduced into 50 ml of n-hexane (Aldrich). 1.8 ml of a 1M triethylaluminum solution in n-hexane was added thereto. The catalyst system in hexane was introduced into the reactor. The reaction system was heated to 60° C. with stirring and pressurized to 23 bar with ethylene for 1 hour. After depressurization, the product was collected in an adjacent vessel. The yield of 1-butene was 90%, expressed ...
Embodiment 3
[0118] Example 3 shows a catalyst system comprising titanate, ether, methylaluminoxane, and optionally a second aluminum compound, and its use in the production of polymers from olefins, especially ethylene. Uses in methods of polyethylene.
[0119] Example 3a. The reaction was carried out in a batch reactor (Parr 300ml autoclave model 4566 Mini Bench Reactor) at 60°C and 23 bar. This temperature and pressure is maintained in the reactor throughout the reaction. 0.25 ml of tetra-n-butyl titanate (DorfKETAL) and 0.25 ml of tetrahydrofuran (Aldrich) were introduced into 50 ml of n-hexane (Aldrich). 1.8 ml of a 1M solution of methylalumoxane in n-heptane (MAO) was added thereto. The catalyst system in hexane was introduced into the reactor. The reaction system was heated to 60° C. with stirring and pressurized to 23 bar with ethylene for 1 hour. After depressurization, the product was collected in an adjacent vessel. The yield of polymer was 95%, expressed as a percentage ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com