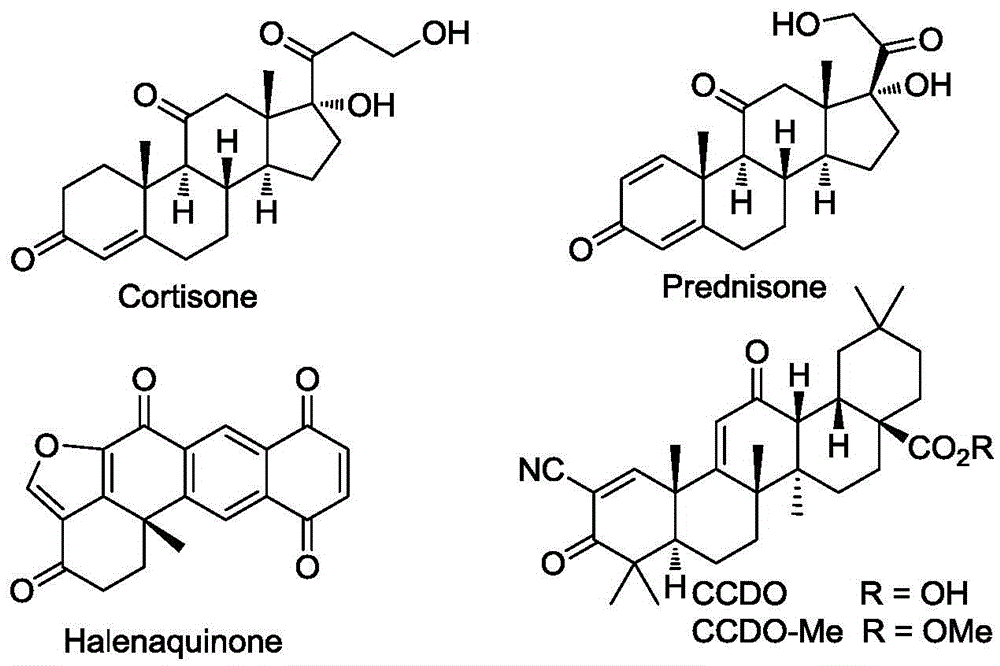
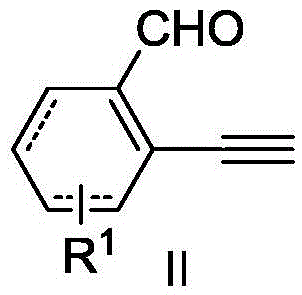
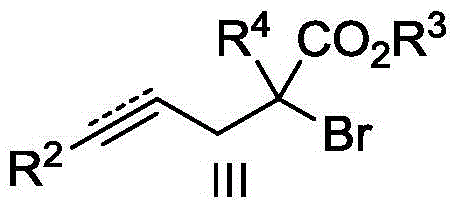
Polycyclic ketone preparation method
A technology of polycyclic ketones and structural formulas, applied in the field of preparation of polycyclic ketones, can solve the problems of insufficient atom economy and long steps, and achieve the effect of large theoretical innovation value, short steps and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take a dry Sis reaction tube, weigh into Cu(OAc) 2 (4.5mg, 0.025mmol), potassium carbonate (35.0mg, 0.25mmol), diethyl azodicarboxylate (8.7mg, 0.050mmol), and the ligand pentamethyldiethylenetriamine (8.7mg, 0.050 mmol), vacuumize and change nitrogen, replace three times, then add structural formula such as 2-ethynyl benzaldehyde (32.5mg, 0.25mmol) shown in 1a and structural formula such as 2-allyl-2 bromomalonic acid shown in 2a Diethyl ester (83.4 mg, 0.30 mmol) in 3 mL of acetonitrile was reacted under reflux at 80°C for 10 h, cooled to room temperature, quenched with 10 mL of water, extracted three times with ethyl acetate (10 mL), combined and washed with saturated drinking water. phase, dried over anhydrous sodium sulfate. The organic phase was concentrated and separated by silica gel (300-400 mesh) column chromatography (eluent: petroleum ether / ethyl acetate volume ratio: 10 / 1) to obtain 63 mg of colorless oily product 3aa, yield 86%. 1 H NMR (400MHz, CDCl 3 ...
Embodiment 2
[0031] Except that enyneal represented by structural formula 1b was used instead of enyneal represented by structural formula 1a in Example 1, the rest of the operation steps were the same as in Example 1. Yield: 76%, colorless liquid. 1 H NMR (600MHz, CDCl 3 )δ1.27(m,6H),2.12(dd,J=8.4,13.3Hz,1H),2.49(dd,J=13.9,15.8Hz,1H),2.90-3.10(m,2H),3.42-3.59 (m,1H),3.91(s,3H),4.13-4.26(m,3H),6.48(d,J=2.3Hz,1H),7.72-7.80(m,1H),8.14-8.18(m,1H ),8.58-8.61(m,1H); 13 C NMR (151MHz, CDCl 3 )δ13.9, 13.9, 39.1, 42.0, 45.3, 52.2, 61.8, 61.9, 66.3, 125.0, 125.7, 128.8, 130.5, 130.9, 133.9, 138.6, 142.8, 165.8, 169.5, 170.5, 195.8; for C 21 h 23 o 7 (M+H) + 387.1444,found 387.1437.
[0032] The reaction formula is as follows:
[0033]
Embodiment 3
[0035] Except that enyneal represented by structural formula 1c is used instead of enyneal represented by structural formula 1a in Example 1, the rest of the operation steps are the same as in Example 1, yield: 75%, white solid, melting point: 97-99°C; 1 H NMR (600MHz, CDCl 3 )δ1.32(dt, J=7.1, 14.4Hz, 6H), 2.18(dd, J=8.3, 13.3Hz, 1H), 2.54(dd, J=13.9, 15.9Hz, 1H), 3.10(m, 2H ), 3.57(tdd, J=2.5, 8.0, 13.8Hz, 1H), 4.26(m, 4H), 6.52(d, J=2.4Hz, 1H), 7.82(dt, J=4.9, 8.3Hz, 2H) ,8.31(s,1H); 13 C NMR (151MHz, CDCl 3 )δ14.0, 14.0, 39.1, 42.1, 45.3, 62.0, 62.0, 66.4, 123.5 (q, J = 272.7Hz), 124.7 (q, J = 3.9Hz), 125.2, 126.3, 129.8 (q, J = 3.4 Hz), 131.1 (q, J=3.4Hz), 131.2, 138.0, 142.4, 169.6, 170.6, 195.5. 19 F NMR (565MHz, CDCl 3 )δ-63.00(s,1H); HRMS(APCI) calcd for C 20 h 20 f 3 o 5 (M+H) + 397.1263,found 397.1255.
[0036] The reaction formula is as follows:
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com