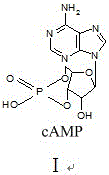
Preparation method of cyclic adenosine monophosphate
A cyclic adenosine monophosphate and reaction technology, applied in the field of preparation of cyclic adenosine monophosphate, can solve the problems of high environmental pressure, long production cycle, low product yield, etc. rate increase effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
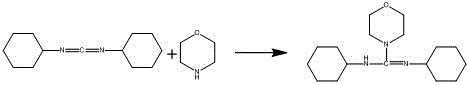
[0031] The preparation of embodiment 1 double salt
[0032]
[0033] Dissolve DCC (26.6g, 100mmol) and morpholine (8.7g, 100mmol) in 100ml of ethanol, heat to dissolve, and then slowly cool down to 0-5°C. Filter, wash with a small amount of ethanol, and dry to obtain a double salt (26.37g, 90% yield), with a melting point of 104.2°C to 105.1°C.
Embodiment 2
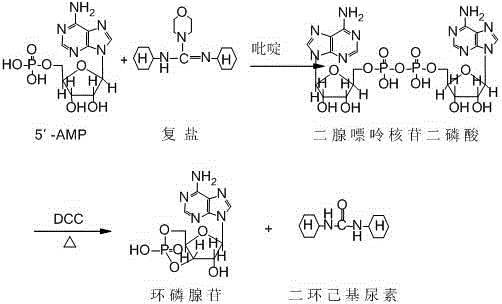
[0034] The preparation of embodiment 2 cyclic adenosine monophosphate
[0035] Put 3.0g of adenosine acid, 2.73g of double salt, and 4.5g of DCC (2.5 chemical equivalents) into dissolved pyridine (500ml), raise the temperature to 110°C, and keep it warm for 3 hours to obtain a reaction dropwise solution. Add 4.5g (2.5 chemical equivalents) of DCC to 500ml of pyridine, heat up to boiling, add the above dropwise solution to the reaction, and the addition time is 2 hours. After the addition, keep the boiling point temperature and continue the reaction for 3 hours. Remove the solvent by distillation under reduced pressure. Add water (500ml) and diethyl ether (500ml) to wash, separate the layers, adjust the pH of the water layer to 1.5-2.0, filter, dry and recrystallize to obtain cyclic adenosine monophosphate 1.2g, with a total yield of 40% and a purity of 99.68%.
[0036] Spectral data: MS (ESI): 328.20
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com