Polythiophene with side chain containing hydrophilic group, and preparation method thereof
A technology of hydrophilic group and polythiophene, which is applied in the field of polythiophene compounds, can solve the problems of low bromination yield and harsh polymerization reaction conditions in the later stage, and achieve good heat resistance, good interface compatibility, and increased migration probability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthetic route of the polythiophene that side chain contains alkoxy group is as follows:
[0024]
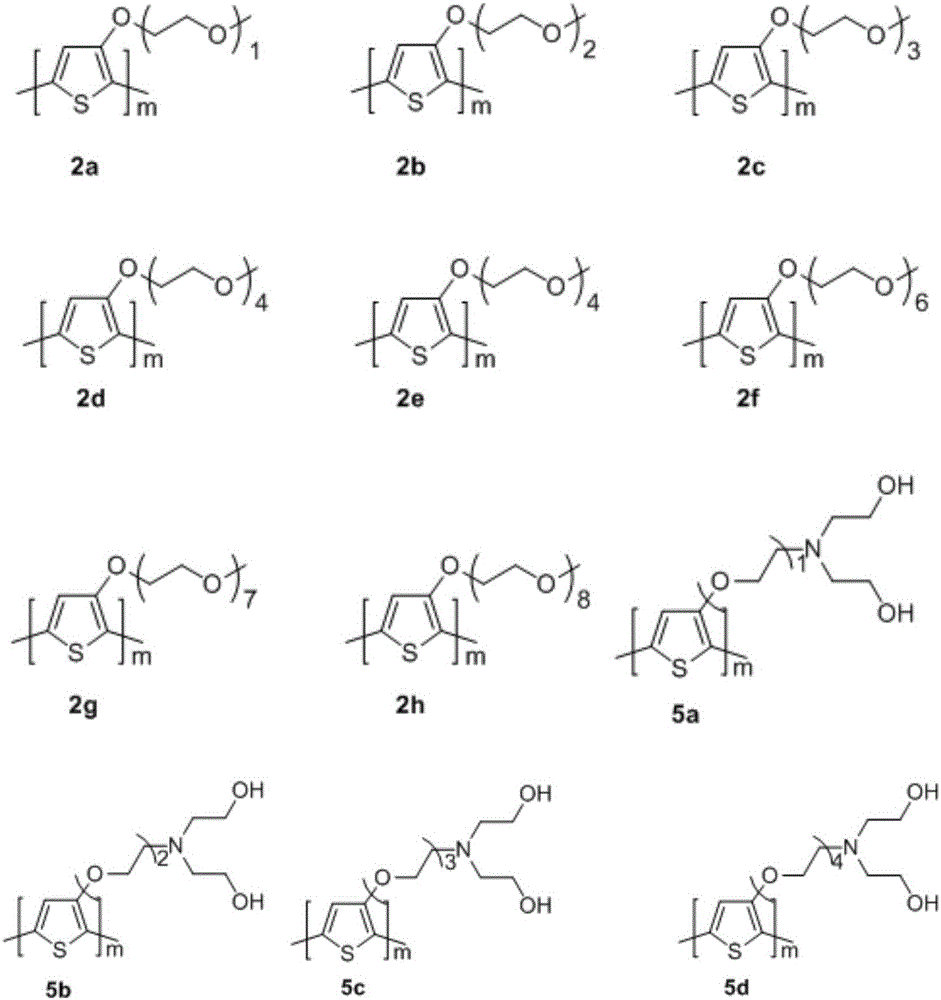
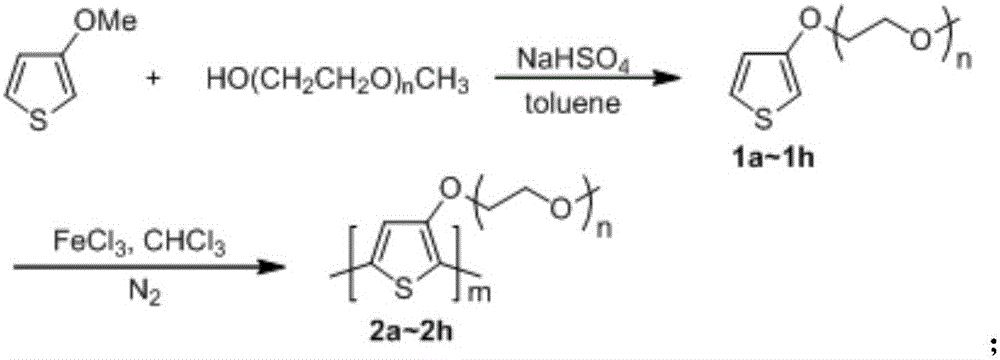
[0025] The overall process is: 3-methoxythiophene in NaHSO 4 As a catalyst, toluene reacts with long-chain alcohol methyl ethers such as ethylene glycol monomethyl ether, diethylene glycol monomethyl ether, triethylene glycol monomethyl ether, tetraethylene glycol monomethyl ether, etc. under the condition of solvent reflux to obtain 3- Alkoxy-substituted thiophene monomers 1a-1h. The series of monomers were in chloroform, anhydrous FeCl 3 As an oxidizing agent, nitrogen is used as a protective gas, and the polythiophene compounds 2a-2h having side chains containing alkoxy groups are obtained by polymerization at a temperature ranging from -10° C. to room temperature.
[0026] Synthesis of Compound 1a 3-(2-Methoxy)ethoxythiophene
[0027] 3-methoxythiophene 5mmol, ethylene glycol monomethyl ether 10.5mmol, NaHSO 4 2mmol and 25mL of toluene were added into a 2...
Embodiment 2
[0054] The synthetic route of the polythiophene that side chain has alcoholamine group is as follows:
[0055]
[0056] The overall process is: 3-methoxythiophene in NaHSO 4 As a catalyst, under the condition that toluene is the solvent and refluxes, coupling occurs with monohalogenated glycols such as 2-chloroethanol, 2-(2-chloroethoxy)ethanol, 2-bromoethanol, 2-(2-bromoethoxy)ethanol, etc. reaction to obtain alkoxy-substituted polythiophene monomers whose end groups are chlorine or bromine; then, the series of thiophene derivatives in K 2 CO 3 Under the conditions of heating and refluxing in an organic solvent such as N,N-dimethylformamide and other alkalis as catalysts, react with diethanolamine respectively to obtain thiophene monomers 4a-4d whose side chain end groups are diethanolamino groups; finally, In chloroform, anhydrous FeCl 3 As an oxidizing agent, polythiophene with side chains having alcoholamine groups can be obtained by polymerizing at -10°C to room tem...
Embodiment 3
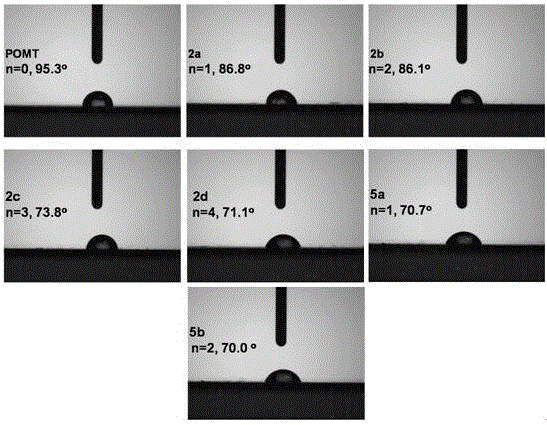
[0083] The side chains prepared in Example 1 and Example 2 contain alkoxy groups and the side chain end groups are alcohol aminopolythiophene compounds for contact angle testing. The test method is: the polymer is configured to have a concentration of 10 -3 mg / mL chloroform solution, and then spin-coated on a clean polished silicon wafer by spin coating method (1000rpm, 30s), and the silicon wafer was placed in a vacuum oven to dry for 24 hours. The water droplet was dropped on the polymer film by the fixed drop method, and then the contact angle between the water droplet and the polymer film was measured by the goniometric method. The result is as figure 1 As shown, the contact angle of poly(3-methoxy)thiophene (POMT) is 95.3°, and the contact angle greater than 90° indicates that the surface of the film is a hydrophobic surface. However, the film contact angles of polythiophenes 2a, 2b, 2c, 2d, 5a and 5b synthesized by the present invention are all less than 90°, indicating...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com