A method for recovering and treating sulfur dioxide and simultaneously producing hydrogen
A sulfur dioxide, recovery and treatment technology, applied in chemical instruments and methods, gas treatment, separation methods, etc., can solve the problems of equipment corrosion and high desulfurization costs, and achieve the effects of suitable reaction conditions, strong operability, and improved removal efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
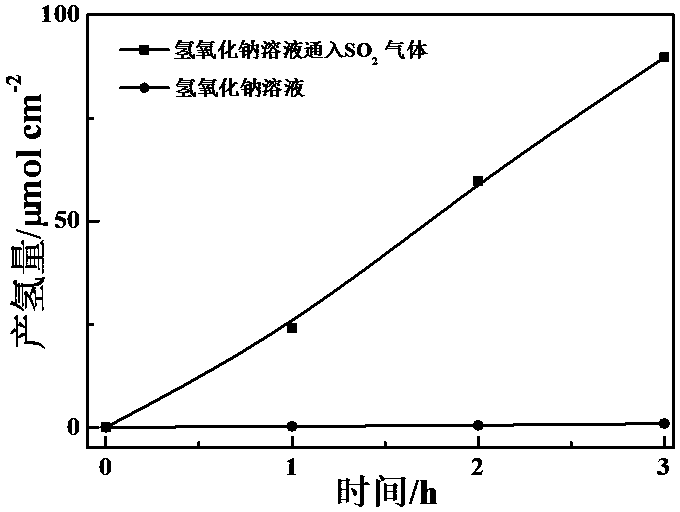
[0021] A. Catalyst preparation: 0.5 mmol Bi(NO 3 ) 3 • 5H 2 O with 0.5 mmol NH 4 VO 3 dissolved in nitric acid solution (V 硝酸 :V 水 =1), and adding absolute ethanol to obtain a bismuth vanadate precursor solution. The bismuth vanadate precursor solution was loaded on the surface of FTO conductive glass by drop coating method. After drying at 60 °C, calcined at 350 °C for 1 h with a heating rate of 5 °C min -1 , after naturally cooling down to room temperature, a bismuth vanadate catalyst is obtained;
[0022] B. In the photocatalytic device, the concentration is 0.0001 mol L -1 Sodium hydroxide solution is used as electrolyte solution, platinum wire electrode is used as cathode, and FTO conductive glass loaded with bismuth vanadate is used as photoanode. Pass 1 ppm of sulfur dioxide gas into the device. Under the action of photoelectrocatalysis, the amount of hydrogen produced in the above experiment was tested.
Embodiment 2
[0024] A. Catalyst preparation: 0.001mmol Bi(NO 3 ) 3 • 5H 2 O with 0.001 mmol NH 4 VO 3 dissolved in nitric acid solution (V 硝酸 :V 水 =3), and adding absolute ethanol to obtain a bismuth vanadate precursor solution. The bismuth vanadate precursor solution was loaded on the surface of FTO conductive glass by drop coating method. After drying at 40 °C, it was calcined at 500 °C for 4 h with a heating rate of 1 °C min -1 , after naturally cooling down to room temperature, a bismuth vanadate catalyst is obtained;
[0025] B. In the photocatalytic device, the addition concentration is 0.75 mol L -1 Sodium hydroxide solution is used as electrolyte solution, platinum wire electrode is used as cathode, and FTO conductive glass loaded with bismuth vanadate is used as photoanode. Pass 200 ppm of sulfur dioxide gas into the device. Under the action of photoelectrocatalysis, the amount of hydrogen produced in the above experiment was tested.
Embodiment 3
[0027] A. Catalyst preparation: 20mmol Bi(NO 3 ) 3 • 5H 2 O with 20mmol NH 4 VO 3 dissolved in nitric acid solution (V 硝酸 :V 水 =5), and adding absolute ethanol to obtain a bismuth vanadate precursor solution. The bismuth vanadate precursor solution was loaded on the surface of FTO conductive glass by drop coating method. After drying at 100 °C, it was calcined at 600 °C for 3 h with a heating rate of 2 °C min -1 , after naturally cooling down to room temperature, a bismuth vanadate catalyst is obtained;
[0028] B. In the photocatalytic device, the addition concentration was 5 mol L -1 Sodium hydroxide solution is used as electrolyte solution, platinum wire electrode is used as cathode, and FTO conductive glass loaded with bismuth vanadate is used as photoanode. Pass 500 ppm of sulfur dioxide gas into the device. Under the action of photoelectrocatalysis, the amount of hydrogen produced in the above experiment was tested.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com