Electrolyte corrosion inhibitor, aluminum-air battery electrolyte and preparation method of aluminum-air battery electrolyte
An aluminum-air battery and corrosion inhibitor technology, which is applied in the direction of fuel cell half-cells and primary battery-type half-cells, can solve the problems of reducing the activity of aluminum anodes, single functions, and reduced corrosion inhibition effects, and achieve corrosion inhibition Good effect, dense deposition layer, improved deposition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
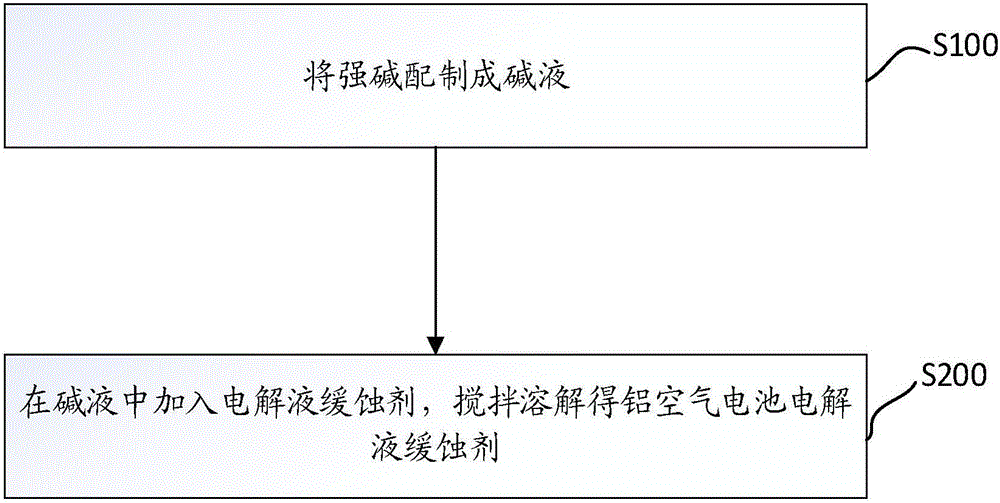
[0039] like figure 1 As shown, this embodiment also provides a method for preparing an aluminum-air battery electrolyte, including the following steps:
[0040] Step S100: providing or preparing a strong alkali solution.
[0041] Wherein, the strong alkali solution can be potassium hydroxide solution or sodium hydroxide solution, with a concentration of 1 to 8 mol / L, preferably 3.5 to 5.5 mol / L;
[0042] Step S200: Add an electrolyte corrosion inhibitor into the strong alkali solution, and mix well to obtain an aluminum-air battery electrolyte.
[0043] As a preferred solution, step S100 can adopt the following specific steps:
[0044] Step S110: preparing sodium hydroxide into a sodium hydroxide solution.
[0045] The concentration of the sodium hydroxide solution is prepared to be 1-8 mol / L, preferably 3.5-5.5 mol / L.
[0046] Step S120: adding powdered aluminum hydroxide to the sodium hydroxide solution, stirring under the condition of heating in a constant temperature w...
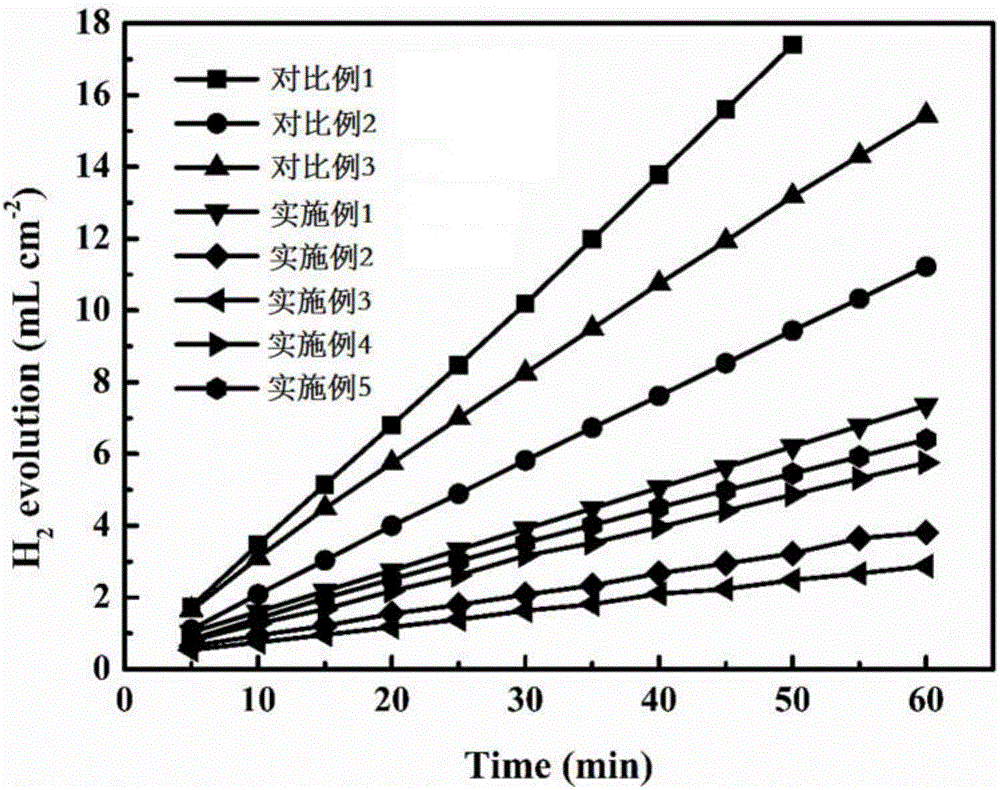
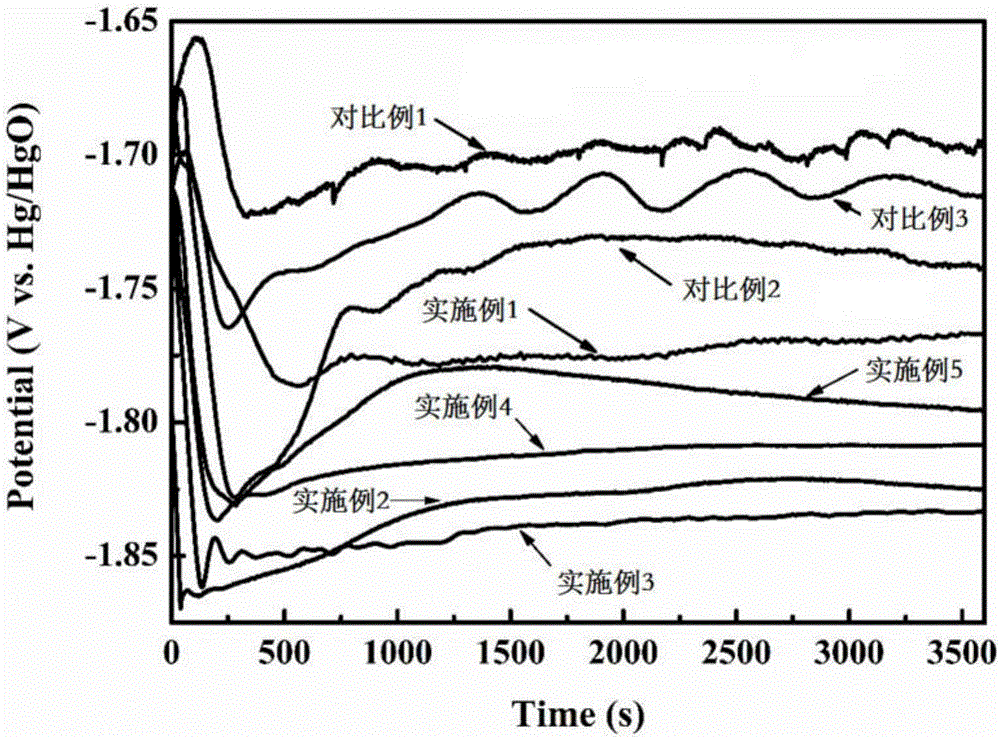
Embodiment 1
[0060] Electrolyte corrosion inhibitors include inorganic phase-forming corrosion inhibitors and organic adsorption corrosion inhibitors. Alkyl dimethyl benzyl ammonium bromide 0.0008mol. Prepare 1L of sodium hydroxide solution with a concentration of 4mol / L, add 40g of aluminum hydroxide powder to the sodium hydroxide solution as a seed crystal, and stir evenly under the condition of heating in a constant temperature water bath at 60°C to obtain lye. Add the ground and mixed electrolyte corrosion inhibitor into the lye, add it in 5 times, and wait for the electrolyte corrosion inhibitor to dissolve under the condition of heating in a constant temperature water bath at 60°C to obtain an aluminum-air battery electrolyte.
Embodiment 2
[0062] Electrolyte corrosion inhibitors include inorganic phase-forming corrosion inhibitors and organic adsorption corrosion inhibitors. Triazole 0.005mol, aspartic acid 0.02mol, cetyltrimethylammonium chloride 0.0005mol. Prepare 1 L of sodium hydroxide solution with a concentration of 4 mol / L, add 40 g of aluminum hydroxide powder as a seed crystal into the sodium hydroxide solution, and stir evenly to obtain lye. Add the ground and mixed inorganic phase-forming corrosion inhibitor into the lye, and add it in 5 times. After the inorganic phase-forming corrosion inhibitor dissolves, add the fully ground and mixed organic adsorption-type corrosion inhibitor, and stir to dissolve. Aluminum-air battery electrolyte. The stirring process in the above preparation process is all completed under the condition of heating in a constant temperature water bath at 50°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com