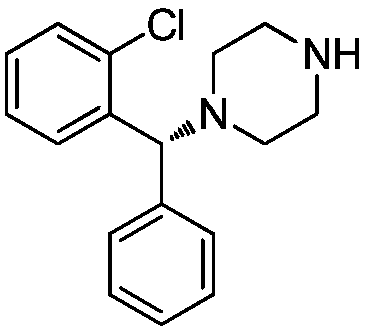
A kind of resolution method of (r)-1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine
A chlorophenyl and phenyl technology, applied in the field of chiral drug impurity preparation, can solve the problems of unavailability and poor splitting effect, and achieve the effects of short reaction time, low environmental pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
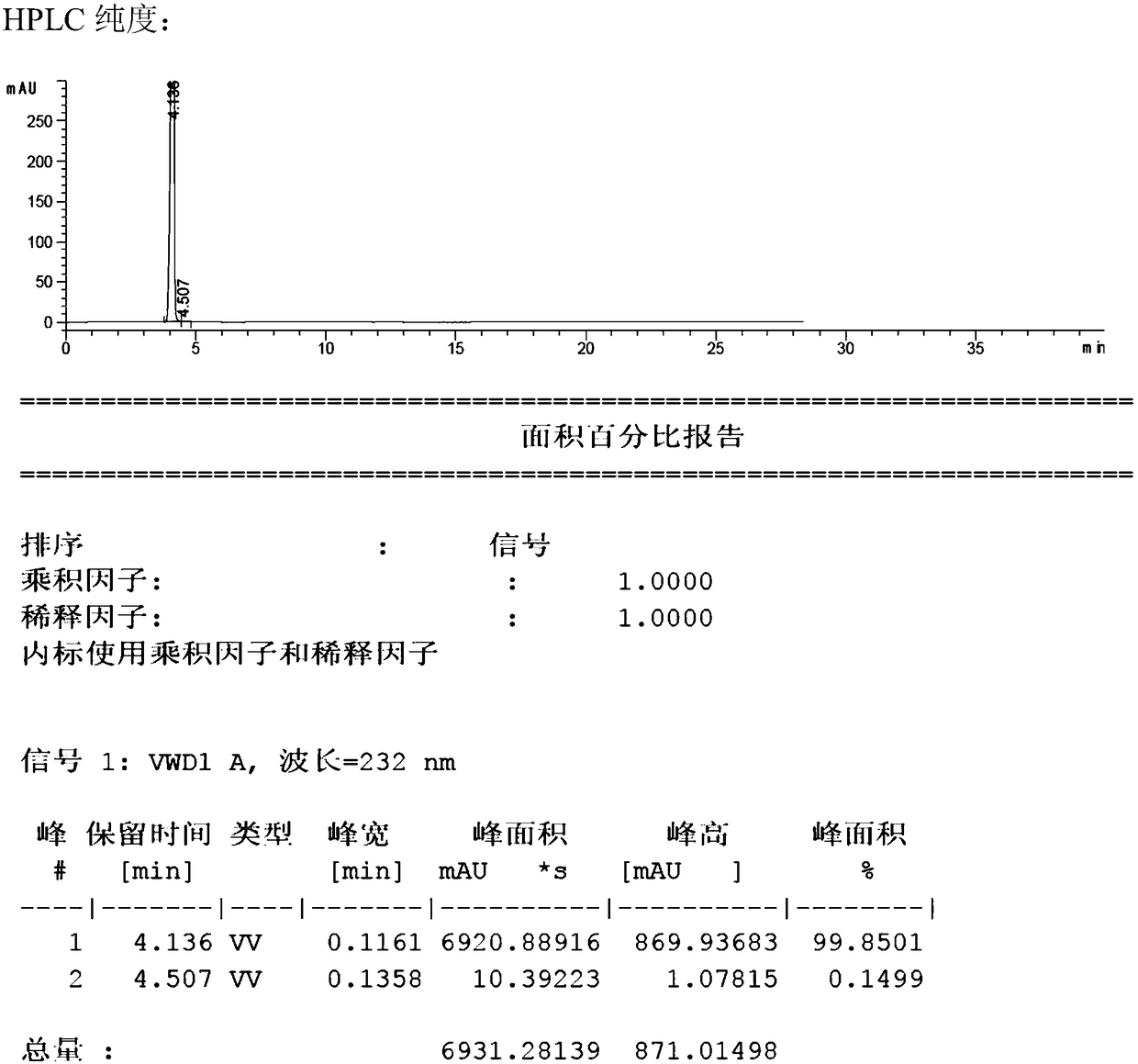
[0018] Racemic 1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine (0.1mol, 28.6g) and resolving agent (S)-(-)-alpha-methylbenzyl isocyanate (0.15mol, 22.0g) was dissolved in 558ml of acetone, and reacted for 2h at 25°C to produce (R)-1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine-(S)-( Precipitation of -)-alpha-methylbenzyl isocyanate, filtered and washed with acetone, yielded (R)-1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine-(S) -(-)-alpha-methylbenzyl isocyanate 19.9g, add water to dissolve, adjust pH=8.0 with saturated sodium carbonate solution, add ethyl acetate to extract, dry, filter, concentrate to obtain (R)-1-((2- Chlorophenyl)-(phenyl)-methyl)-piperazine 12.9 g. The total yield is 45%, melting point: 91-93°C, specific rotation [α] D It is -21.8° (methanol), the purity is 99.85%, and the ee value is 99.5%.
[0019] 1 H-NMR (CDCl 3 ):7.37-7.18(m,9H),4.18(s,1H),2.88(q,J=6Hz,4H),2.34(s,4H),2.19(s,1H).
[0020] 13 C-NMR (CDCl 3 ):142.1,141.2,132.4,129.1,129.0,128....
Embodiment 2
[0030] Racemic 1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine (0.1mol, 28.6g) and resolving agent (S)-(-)-alpha-methylbenzyl isocyanate (0.1mol, 14.7g) was dissolved in 558ml of acetone, and reacted for 2h at 25°C to produce (R)-1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine-(S)-( Precipitation of -)-alpha-methylbenzyl isocyanate, filtered and washed with acetone, yielded (R)-1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine-(S) -(-)-alpha-methylbenzyl isocyanate 17.8g, add water to dissolve, adjust pH=8.0 with saturated sodium carbonate solution, add ethyl acetate to extract, dry, filter, concentrate to obtain (R)-1-((2- Chlorophenyl)-(phenyl)-methyl)-piperazine 11.5 g. The total yield is 40.2%, melting point: 91-93°C, specific rotation [α] D It is -21.5° (methanol), the purity is 99.53%, and the ee value is 99.1%.
Embodiment 3
[0032] Racemic 1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine (0.1mol, 28.6g) and resolving agent (S)-(-)-alpha-methylbenzyl isocyanate (0.2mol, 29.4g) was dissolved in 558ml of acetone, and reacted for 2h at 25°C to produce (R)-1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine-(S)-( Precipitation of -)-alpha-methylbenzyl isocyanate, filtered and washed with acetone, yielded (R)-1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine-(S) -(-)-alpha-methylbenzyl isocyanate 20.8g, add water to dissolve, adjust pH=8.0 with saturated sodium carbonate solution, add ethyl acetate to extract, dry, filter, concentrate to obtain (R)-1-((2- Chlorophenyl)-(phenyl)-methyl)-piperazine 13.6 g. The total yield is 47.5%, melting point: 91-93°C, specific rotation [α] D It is -21.5° (methanol), the purity is 99.50%, and the ee value is 99.0%.
[0033] By comparing Example 1 with Example 2 and Example 3, we found that racemic 1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine and (S)-(-)-alpha -The reso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com