Preparation method of ion exchange membrane used in vanadium battery
An ion exchange membrane, vanadium battery technology, applied in fuel cells, regenerative fuel cells, circuits, etc., can solve the problems of anion exchange membrane human body and environmental hazards, improve chemical stability and mechanical properties, low vanadium ion permeability , the effect of low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
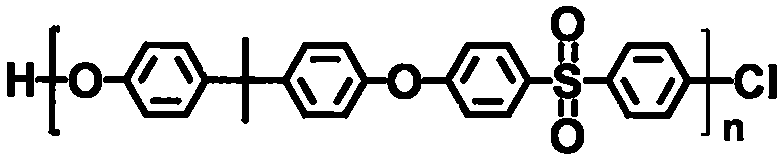
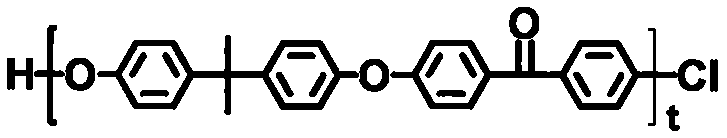
[0030] The embodiment of the present invention discloses a preparation method of an ion exchange membrane for a vanadium battery, comprising the following steps: adding tetrabutylammonium bromide, an aqueous solution of sodium hydroxide and 1,4-butanesulfonic acid to the DMSO solution of the polycondensate The DMSO solution of the lactone is stirred for 5-8 hours under a nitrogen atmosphere, filtered, washed, recrystallized, and dried to obtain a sulfonated polycondensate, which is selected from one or more of formula 1 and formula 2 ,
[0031]
[0032] Formula 1),
[0033]
[0034] Formula 2,
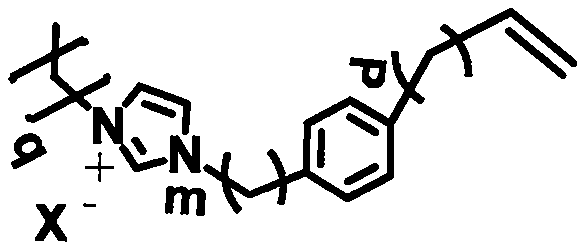
[0035] Wherein, wherein, any integer in n=24-36, any integer in t=25-36; the polymerization type imidazolium salt ionic liquid monomer, styrene and initiator are mixed, heated under the protection of inert gas React for 1-3 hours, precipitate, wash, dry, and then dissolve in N,N-dimethylformamide to obtain a 2%-5% solution, drop it on a polytetrafluoroethylene plate, and obtain...
Embodiment 1
[0057] (1) Sulfonation of polycondensate: the polycondensate shown in 100g formula 1 is dissolved in 1.5L DMSO solution, and 4g tetrabutylammonium bromide is added therein, the aqueous solution (concentration 50wt%) of 150mL sodium hydroxide and 625mL DMSO solution of 1,4-butane sultone (concentration 2mol / L,), the above mixture was stirred at room temperature under nitrogen atmosphere for 5 hours, the product was filtered, washed with acetone and ethanol, recrystallized, dried and other steps. get,
[0058]
[0059] Formula 1)
[0060] Wherein, any integer in n=24-36;
[0061] (2) Polymer addition synthesis: in the reaction vessel, add the mixture of the polymeric imidazolium salt ionic liquid monomer shown in 75g formula 3, 25g styrene and 1g azobisisobutyronitrile (AIBN), after feeding Under the protection of an inert gas, heat the reaction for 3 hours to obtain the product; the obtained product is precipitated, washed, and dried in a vacuum oven at 80°C for 10 hours, ...
Embodiment 2
[0066] (1) Sulfonation of polycondensate: the polycondensate shown in 100g formula 2 is dissolved in 1.5L DMSO solution, and 4g tetrabutylammonium bromide is added therein, the aqueous solution (concentration 50wt%) of 150mL sodium hydroxide and 625mL DMSO solution of 1,4-butane sultone (concentration 2mol / L,), the above mixture was stirred at room temperature under nitrogen atmosphere for 5 hours, the product was filtered, washed with acetone and ethanol, recrystallized, dried and other steps. get,
[0067]
[0068] Formula 2)
[0069] Wherein, t=any integer in 25-36. ;
[0070] (2) Polymer addition synthesis: in the reaction vessel, add the mixture of the polymeric imidazolium salt ionic liquid monomer shown in 75g formula 3, 25g styrene and 1g azobisisobutyronitrile (AIBN), after feeding Under the protection of an inert gas, heat the reaction for 3 hours to obtain the product; the obtained product is precipitated, washed, and dried in a vacuum oven at 80°C for 10 hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com