Cuprous selenide cluster supported visible-light-induced catalyst with property of reducing Cr (VI) ions
A catalyst, visible light technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, reduced water/sewage treatment, etc., can solve problems such as Cr(VI) ion pollution, achieve chemical conversion Contaminants are rapid, inexpensive, and cycle-reducing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of cuprous selenide cluster-supported visible light catalyst C with the property of reducing Cr(VI) ions:
[0032] (1) Synthesis of the aromatic ring-containing selenoether ligand L: Take a 100 mL three-necked flask, repeatedly evacuate it with nitrogen for 3 times, measure 60 mL of ethanol, dry it with anhydrous magnesium sulfate, and add it to the three-necked flask. Weigh 0.156g of diphenyl diselenide (0.5mmol) and add it to a three-necked flask filled with nitrogen, repeatedly degas, ice bath for 3-8 minutes, weigh 0.7674g of 20 equivalents of sodium borohydride and add it to a three-necked flask, and the solution Immediately turn into a white emulsion, continue to ice bath for 15min, remove the ice bath, continue to react at room temperature for 2h, the solution gradually becomes a clear and transparent colorless solution, after 2h, weigh 0.098g (0.25mmol) 9-(3 -Bromo-2-bromomethylpropyl)anthracene was dissolved in a little refluxed tetrahydrofuran, and...
Embodiment 2
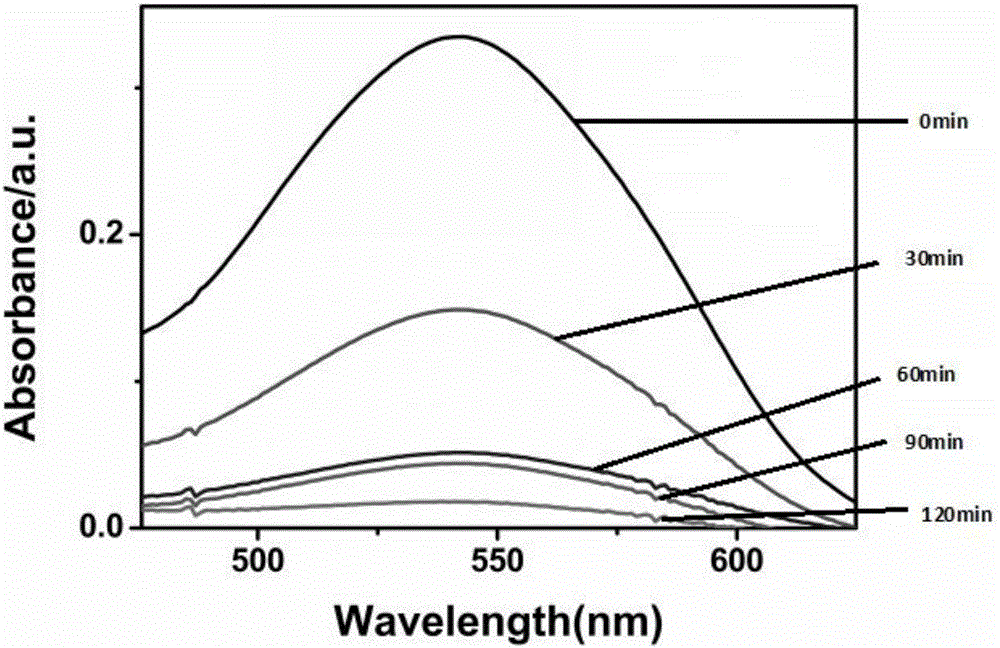
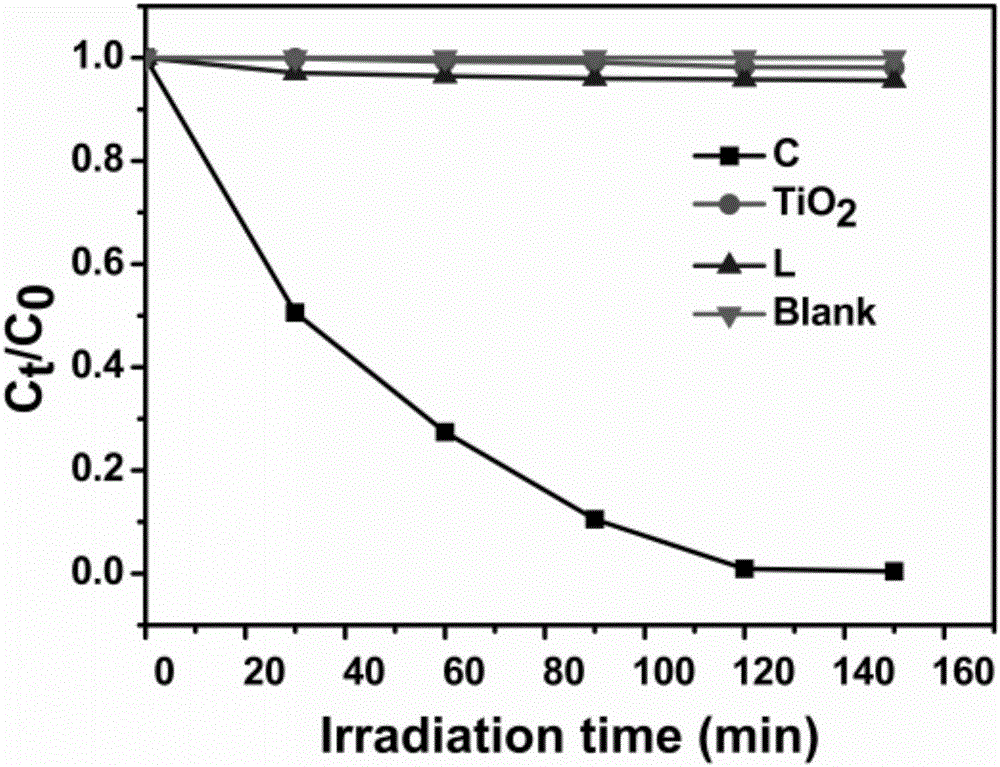
[0038] Prepare 4 groups of 30ml 1×10 -4 mol / L potassium dichromate aqueous solution, add respectively 20mg embodiment 1 and prepare gained catalyst C, TiO 2 , Ligand L and a group of blank experiments, stirred in the dark for 30 minutes and then turned on the light source to continue the reaction. Collect 3ml of reaction liquid samples every 30min, and measure the UV of each group of samples according to the above operation. Such as figure 2 Shown, λ>400nm, C 0 is the initial Cr(VI) concentration, C t It is the concentration of Cr(VI) changing with time. It can be seen from the analysis of the ultraviolet absorption spectrum that after the catalyst C is added, the absorption peak of the Cr(VI) ion at a wavelength of 540nm weakens and disappears with the reaction time, while the other groups do not occur. It can be seen that only catalyst C has visible light catalytic reduction activity.
Embodiment 3
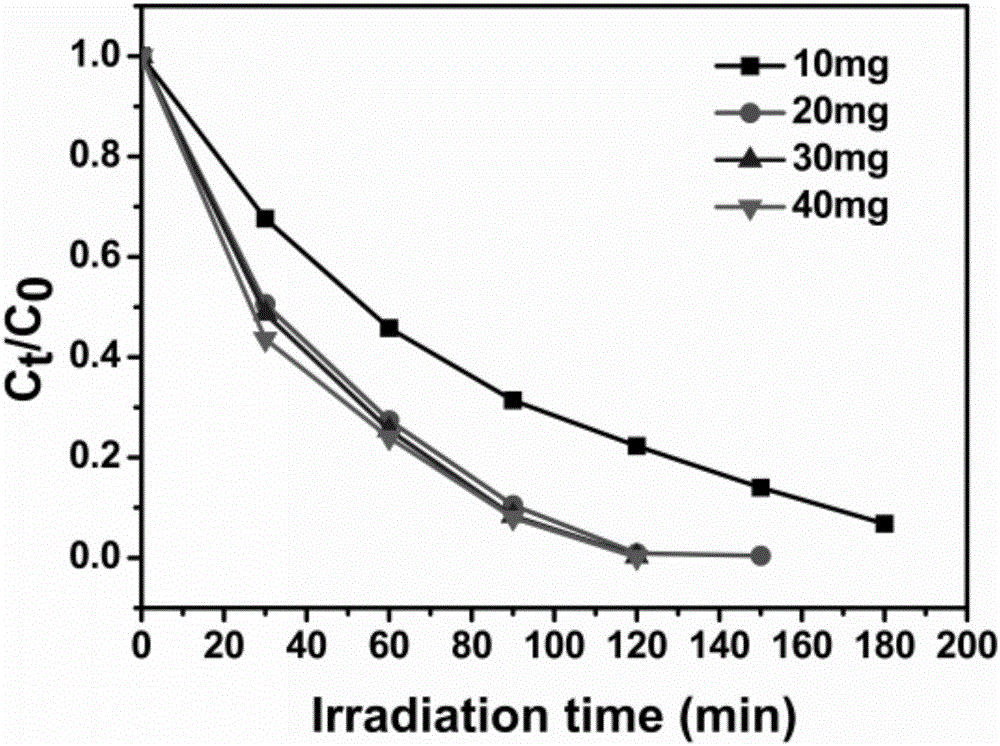
[0040] Prepare 4 groups of 30ml 1×10 -4 10-40 mg of the catalyst C prepared in Example 1 was added to the mol / L aqueous solution of potassium dichromate, stirred in the dark for 30 min, and then the light source was turned on to continue the reaction. Collect 3ml of reaction liquid samples every 30min, and measure the UV of each group of samples according to the above operation. The result is as image 3 shown, where the initial concentration is 1×10 -4 mol / L, analyzing the ultraviolet absorption spectrum, it can be seen that as the input amount of catalyst increases from 10mg-20mg, the catalytic conversion rate increases obviously. When continuing to increase the amount of catalyst input to 40mg, the catalytic reduction time is not shortened, and when the input amount of catalyst is 20 mg, there is the best catalytic conversion efficiency.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com