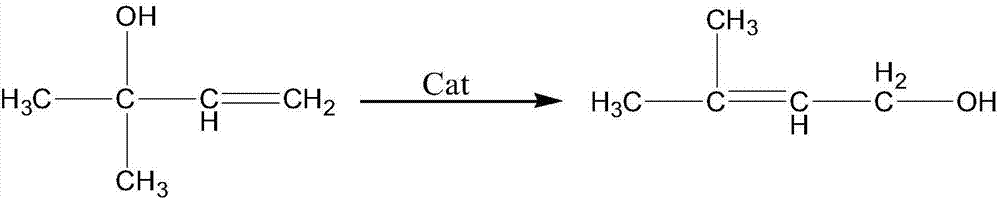
Synthesis of Prenyl Alcohol by Isomerization of Aqueous Methyl Butenol
A water-containing methyl butenol and isopentenol technology, which is applied in the field of chemical engineering, can solve the problems of low selectivity, low reactivity, and increase the separation process, and achieve high reactivity and selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
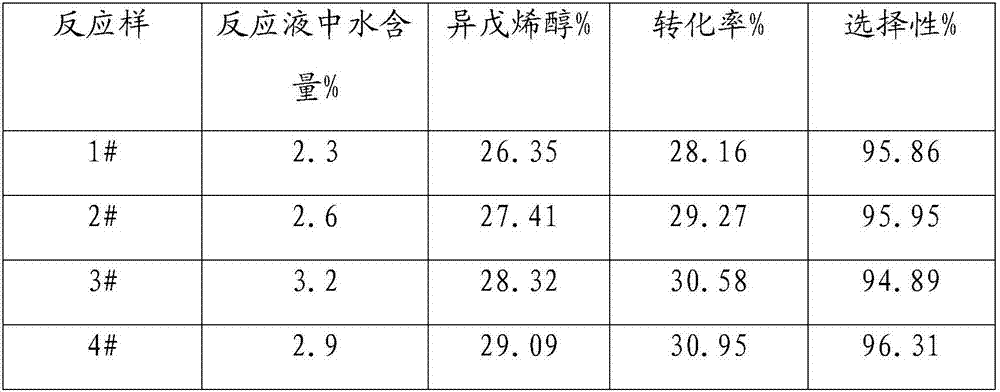
[0019] Embodiment 1-9: Isomerization experiments under different water contents
[0020] Add about 150g of methylbutenol raw material into a 250mL high-pressure stirred tank, and add a certain amount of water to prepare reaction raw materials with different water contents, and then add about 2.0g of ammonium metavanadate. After replacing with nitrogen for several times, the temperature was raised to 160°C, and samples were taken after 60 minutes of reaction. The samples were analyzed by gas chromatography and calculated as shown in Table 1.
[0021] Table 1 Results of isomerization reaction at different water contents
[0022] Example
[0023] Note: Comparative Example 8* uses the ester synthesized from vanadium pentoxide and n-hexanol as the catalyst, and 9* uses the ester synthesized from vanadium pentoxide and isoamyl alcohol as the catalyst. The results show that when esters are used as catalysts, a small amount of water will obviously reduce the selectivity of ...
Embodiment 10-13
[0024] Embodiment 10-13: Isomerization experiments under different catalyst concentrations
[0025] Add about 150g of methylbutenol raw material and 4g of water into a 250mL high-pressure stirred tank to prepare a reaction material with a water content of 2.6%, and then add a certain amount of ammonium metavanadate. After replacing with nitrogen for several times, the temperature was raised to 160°C, and samples were taken after 60 minutes of reaction. The samples were analyzed by gas chromatography and calculated as shown in Table 2.
[0026] Table 2 Experimental results of different catalyst concentrations
[0027] Example
Embodiment 14
[0028] Embodiment 14: Catalyst circulation experiment
[0029] Add about 600g of methylbutenol raw material into a 1000mL high-pressure stirred tank, and then add 15g of water and 15g of ammonium metavanadate. After replacing with nitrogen for several times, the temperature was raised to 160°C, and the reaction was stopped after 60 minutes and samples were taken for analysis. After cooling, the reaction liquid is filtered to obtain the catalyst for use, and the filtrate is rectified to obtain the product isopentenol and unreacted methyl butenol. Add unreacted methylbutenol to 600g and then add it into the reaction kettle to continue the reaction for 60min. The analysis results of the reaction solution circulated four times were calculated and obtained as shown in Table 3. The analytical results of product isopentenol are shown in Table 4.
[0030] Table 3 Results of isomerization cycle experiments
[0031]
[0032] Table 4 Analysis results of product isopentenol composi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com