Application of piperaquine nitrogen oxide in preparation of medicines for resisting malaria
A nitrogen oxide and anti-malarial technology, applied in drug combinations, anti-infectives, and resistance to vector-borne diseases, etc., can solve the problem of slow action of piperaquine, less research on the chemical properties, biological activities and uses of compounds, and hinder the technical field Research and development and other issues to achieve good antimalarial activity and scientific and rigorous experimental design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthesis of piperaquine mono-nitrogen oxide and piperaquine di-nitrogen oxide
[0028] (1) piperazine (4.3g, 5.05mol) and Na 2 CO 3 (4g) was miscible in 20mL ethanol, then added 4-7 dichloroquinoline (5g, 2.525mol) in ethanol (15mL) solution, reacted at 80°C for 3h, and refluxed the reaction solution at room temperature for 3h, after the reaction was completed, the reaction The liquid solvent was evaporated to dryness, water (30mL) was added, then extracted with ethyl acetate (3×15mL), the combined organic layers were washed with Na 2 SO 4 After drying, the organic solvent was evaporated to dryness, and the sample was purified by silica gel column (eluent: methanol, dichloromethane) to obtain compound 3 as a light yellow solid. The synthetic route is as follows:
[0029]
[0030] (2) piperazine (0.04g, 0.2336mmol) and NaHCO 3 (4.0g) was miscible in 30mL ethanol, compound 5 (0.05g, 0.2336mmol) was added, the reaction solution was stirred and re...
Embodiment 2
[0039] Embodiment 2: in vitro activity test
[0040] 1. Test material:
[0041] Plasmodium: International Standard Strain 3D7 of Plasmodium falciparum, cited from ATCC Biological Resource Conservation Center of the United States; infection before experiment
[0042] Rate dilution is 0.5%, 2% blood pressure volume.
[0043] Piperaquine phosphate: purchased from Wuhan Xinjialing Biotechnology Co., Ltd. (batch number: 140305; purity 99.4%).
[0044] Chloroquine phosphate: purchased from China National Institutes for Food and Drug Control (batch number: 100421-200401; purity 98.9%).
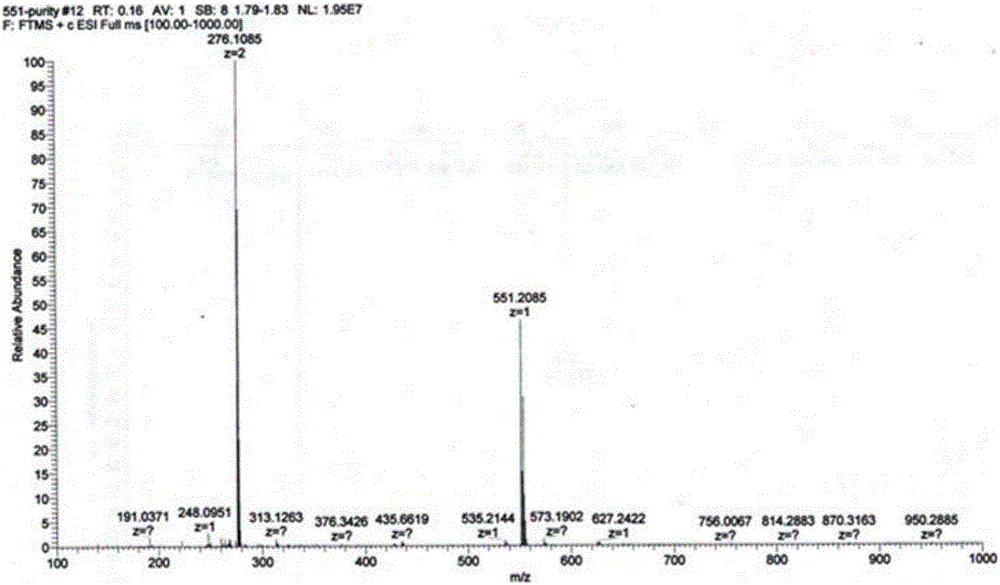
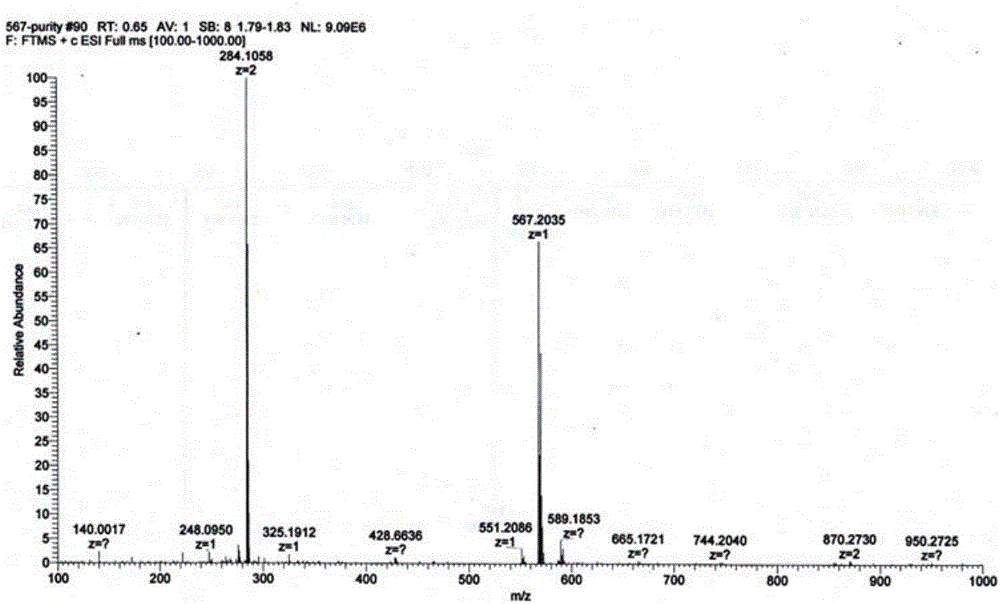
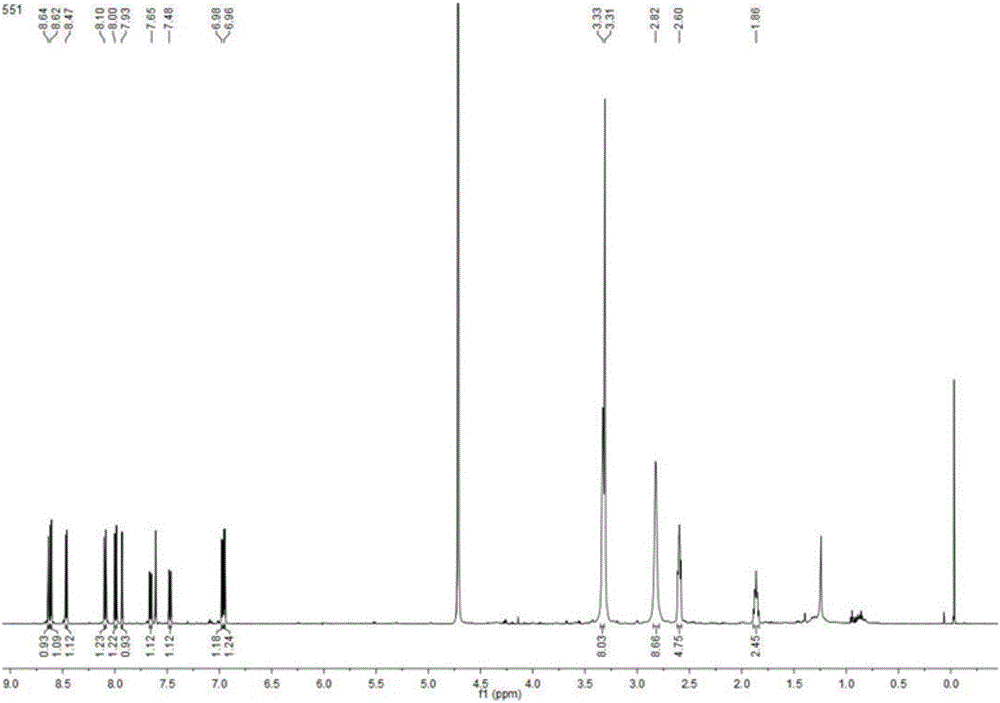
[0045] Piperaquine mono-nitrogen oxide and piperaquine bis-nitrogen oxide: compounds M1 and M2 prepared in Example 1, the structure and purity of which were verified by MS, NMR and HPLC methods (purity>99.0%).
[0046] 2. Test method and grouping:
[0047] (1) Piperaquine phosphate treatment group (PQP group): piperaquine phosphate was prepared with dimethyl sulfoxide (DMSO) as 5 The mol / L stoc...
Embodiment 3
[0058] Embodiment 3: animal experiments
[0059] 1. Experimental materials:
[0060] Experimental animals: ICR mice, weighing 22±2g, purchased from the Experimental Animal Center of Shanxi Medical University.
[0061] Plasmodium falciparum: Plasmodium yoelii strain BY265, cited from ATCC Biological Resource Conservation Center, USA.
[0062] Piperaquine phosphate (PQP): purchased from Wuhan Xinjialing Biotechnology Co., Ltd. (batch number: 140305; purity 99.4%).
[0063] Piperaquine mono-nitrogen oxide M1 and piperaquine bis-nitrogen oxide M2: prepared in Example 1, the structure and purity were verified by MS, NMR and HPLC methods (purity>99.0%).
[0064] 2. Plasmodium falciparum inhibition experiment
[0065] Three dose groups (1, 2, 4 mg / kg) were set up for piperaquine mono-nitrogen oxide M1 and piperaquine bis-nitrogen oxide M2, with 6 mice in each group; the positive control drug was piperaquine phosphate group, and the dose was 4 mg / kg . Mice were injected intraperi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com