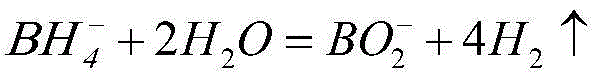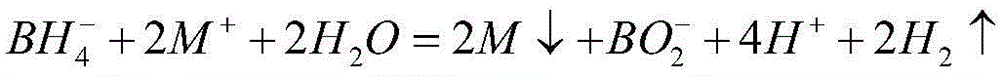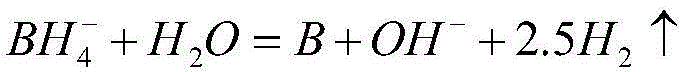Nickel-based amorphous hollow microsphere alloy catalyst, and preparation method and application thereof
A technology of alloy catalysts and amorphous alloys, applied in chemical instruments and methods, physical/chemical process catalysts, hydrocarbon production from oxygen-containing organic compounds, etc., can solve the limitation of heterogeneous catalysis kinetic mass transfer process, reduce catalyst The problems of service life and Ni dispersion are not high, and the effects of reducing thermodynamic diffusion resistance, reducing oxygen content and low density are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Weigh 10.0 g of dodecyl primary amine (DDA) and add it to a mixed solution of 500 ml of deionized water and 200 ml of ethanol, stir and dissolve in a water bath at 30°C, and keep stirring for 60 min to obtain a milky white opaque mixed solution. Then, 37.56 g of tetraethyl orthosilicate (TEOS) was added dropwise into the mixture, and the molar ratio of the final mixed colloid group was 1TEOS:0.30DDA:18.78EtOH:154H2O. The reaction was kept stirring at room temperature for 6 h to obtain a white precipitate. After filtering, washing with deionized water, and drying overnight in a vacuum oven at 55°C, the original powder of mesoporous SiO2 hollow microspheres without further treatment can be obtained. The raw powder was roasted in a muffle furnace, and the temperature was programmed from room temperature to 500 °C in an air atmosphere at a heating rate of 10 °C / min, kept at 500 °C for 4 h, and then naturally cooled to room temperature. The obtained sample was named HSS-1, ...
Embodiment 2
[0043] Weigh 10.0 g of cetyltrimethylammonium bromide (CTAB) and add it to a mixed solution of 400 ml of deionized water and 300 ml of ethanol, stir and dissolve in a water bath at 40°C, and keep stirring for 40 minutes to obtain a milky white opaque mixed solution. Then, 37.56 g of tetraethyl orthosilicate (TEOS) was added dropwise to the mixed solution, and the molar ratio of the final mixed colloid group was 1TEOS:0.26CTAB:28.17EtOH:123H2O. The reaction was kept stirring at room temperature for 7 h to obtain a white precipitate. After filtering, washing with deionized water, and drying in a vacuum oven at 65°C overnight, the mesoporous SiO without further treatment can be obtained. 2 Hollow microsphere powder. The raw powder was roasted in a muffle furnace, and the temperature was programmed from room temperature to 550 °C in an air atmosphere at a heating rate of 10 °C / min, kept at 550 °C for 4 hours, and then naturally cooled to room temperature. The obtained sample was...
Embodiment 3
[0045] Weigh 18.0 g of dodecyldimethyl tertiary amine (DMA12) and add it to a mixed solution of 450 ml of deionized water and 250 ml of ethanol, stir and dissolve in a water bath at 45°C, keep stirring for 30 min, and obtain a milky white opaque mixed solution. Then, 37.56 g of tetraethyl orthosilicate (TEOS) was added dropwise into the mixture, and the molar ratio of the final mixed colloid group was 1TEOS:0.27DMA12:23.48EtOH:138H2O. The reaction was kept stirring at room temperature for 8 h to obtain a white precipitate. After filtering, washing with deionized water, and drying overnight in a vacuum oven at 60°C, the original powder of mesoporous SiO2 hollow microspheres without further treatment can be obtained. The raw powder was roasted in a muffle furnace, and the temperature was programmed from room temperature to 600 °C in an air atmosphere at a heating rate of 10 °C / min, kept at 600 °C for 4 h, and then naturally cooled to room temperature. Obtained SiO 2 The mesopo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com