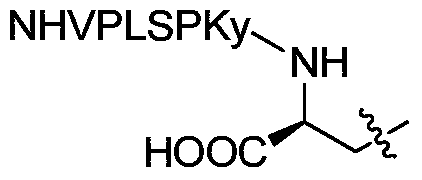
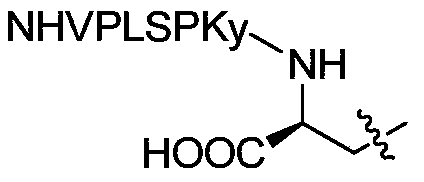
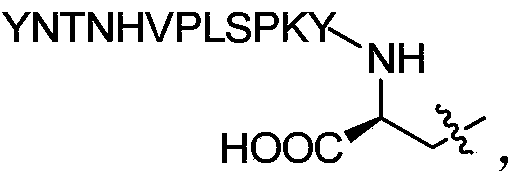<18>F-labeled polypeptide compound, and preparation method and application thereof
A peptide compound, 18F technology, applied in the field of 18F-labeled peptide compound and its preparation, can solve the problems of less imaging probes, long reaction time, low reaction product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] 20mCi 18 f - By quaternary ammonium type anion column QMA (the product of American Waters company, 18 f - Provided by Shanghai Kexing Pharmaceutical Co., Ltd.) after capture, take 1.0mL K2.2.2 (ie Kryptofix 222) solution (14.4mg K2.2.2, 3.0mg K 2 CO 3 , 960μL acetonitrile, 40μL water solution) will 18 f - Rinse into the reaction bottle, immerse the reaction bottle in an oil bath at 95°C, blow dry with nitrogen, then add 500 μL of anhydrous acetonitrile to dry, repeat the above operation twice.
Embodiment 2
[0074] (1) 30mCi 18 f - By quaternary ammonium type anion column QMA (the product of American Waters company, 18 f - Provided by Kexing Pharmaceutical Co., Ltd.) after capture, take 1.2mL K 222 (i.e. Kryptofix 222) solution (17.5mg K 222 , 3.5 mg K 2 CO 3 , 1152μL acetonitrile, 48μL water solution) will 18 Rinse F into the reaction bottle, immerse the reaction bottle in an oil bath at 95°C, dry it with nitrogen, then add 500 μL of anhydrous acetonitrile to dry it, repeat the drying with anhydrous acetonitrile twice;
[0075] (2) 500 μL of anhydrous acetonitrile solution containing 5 mg 2-azidoethyl p-toluenesulfonate was rapidly added to the solution containing K 222 、K 2 CO 3 and 18 f - In the mixture, stop the reaction after closed reaction at 95°C for 10 minutes, and cool in an ice-water bath to obtain compound B1, the labeling rate can reach more than 97%;
[0076]
[0077] (3) Add 200 μL of acetonitrile to the reaction liquid containing compound B1, nitroge...
Embodiment 3
[0082] (1) Add 500 μL of anhydrous acetonitrile solution containing 5 mg of 2-azidoethyl p-toluenesulfonate rapidly to the solution containing K 222 、K 2 CO 3 and 18 f - In the mixture, stop the reaction after closed reaction at 95°C for 10 minutes, and cool in an ice-water bath to obtain compound B1, the labeling rate can reach more than 97%;
[0083]
[0084] (2) Add 200 μL of acetonitrile to the reaction solution containing compound B1, carry out nitrogen gas auxiliary flow, carry out distillation, and collect the condensate, distill for 10-20min to obtain about 700 μL of acetonitrile condensate collection liquid containing purified compound B1, the distillation efficiency Can reach 75%;
[0085] (3) Add 40 μL of 0.45 mol / L copper sulfate solution and 40 μL of 1.5 mol / L sodium ascorbate solution successively into a 1.5 mL finger tube, mix well and add 350 μL of PBS solution containing 2.0 mg of compound D2 (pH = 6.0), after vortex mixing for 1 min, 300 μL of acetoni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com