LiNi0.6-xCo0.2Mn0.2AlxO2-yFy positive electrode material for lithium ion cell and preparation method thereof
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as poor electrochemical performance, improve discharge specific capacity, improve cycle performance, and improve reversible de-intercalation/intercalation capabilities Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
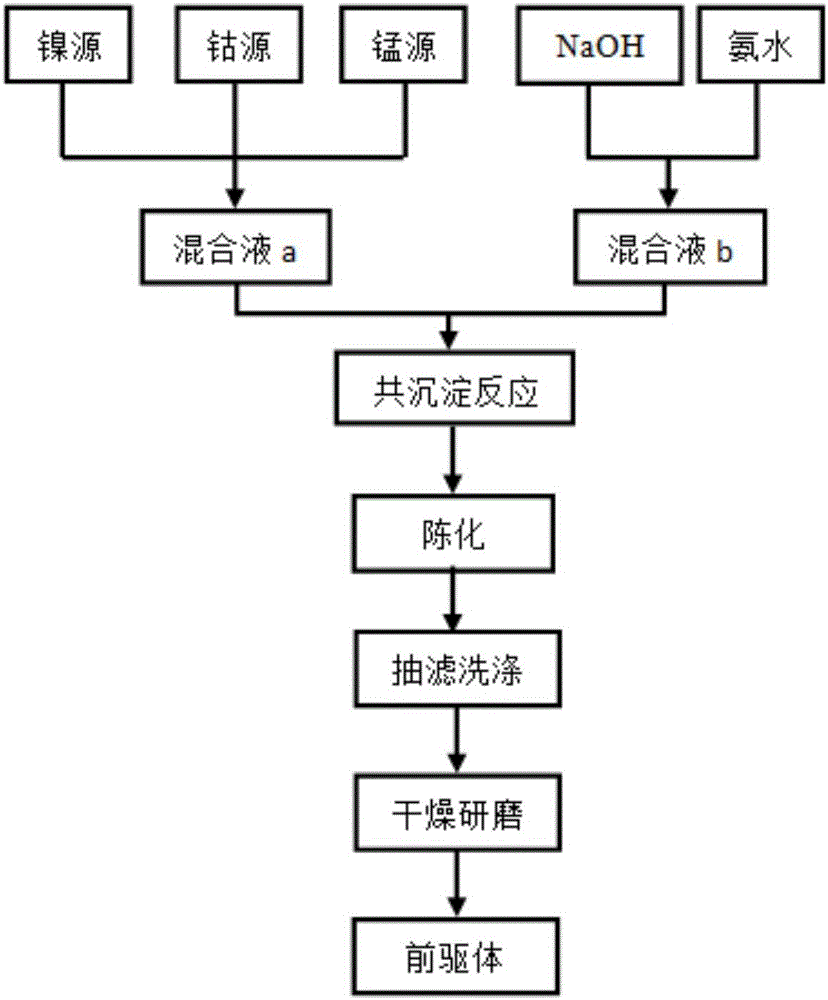
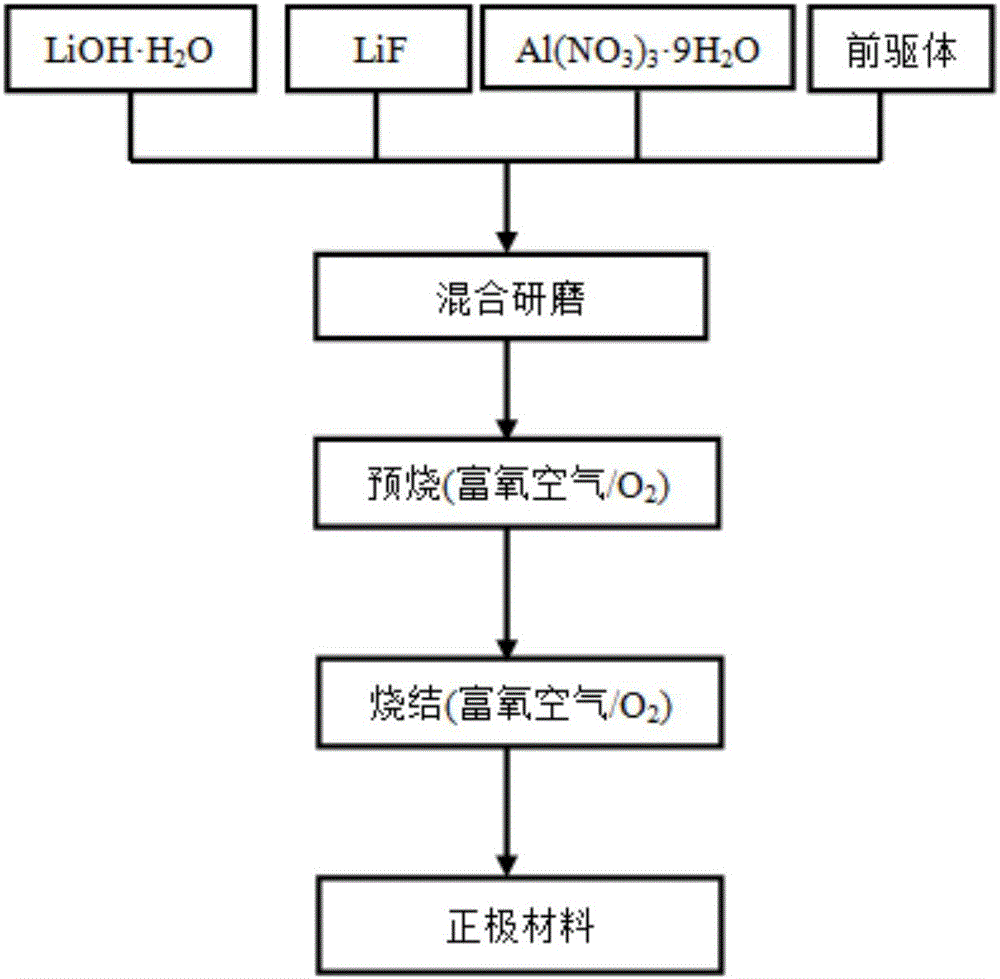
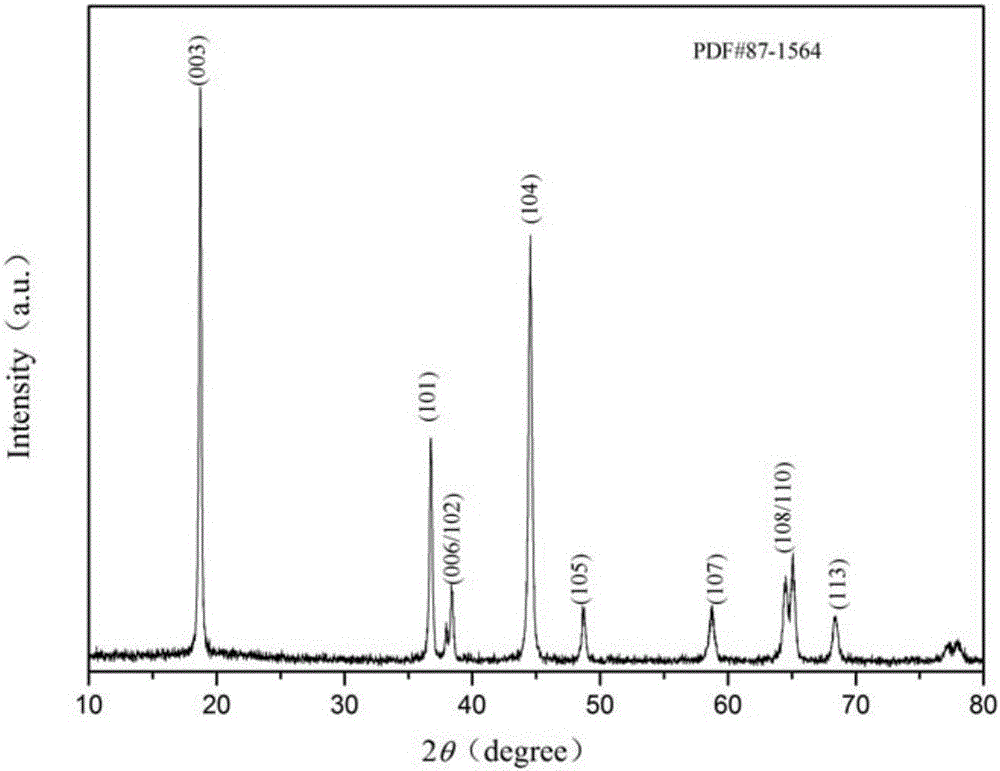
[0036] The present embodiment provides lithium-ion battery cathode material LiNi 0.6-x co 0.2 mn 0.2 Al x o 2-y f y , x=0.01, y=0.02; that is, Al and F doping amounts are 0.01 and 0.02 respectively, and nickel sulfate, cobalt sulfate, and manganese sulfate are weighed according to the molar ratio Ni:Co:Mn=5.9:2:2, and are formulated into 1.0 mol / L sulfate mixed solution a; mix sodium hydroxide solution and ammonia water to form a mixed solution with a concentration of sodium hydroxide of 2.0 mol / L and a concentration of ammonia water of 0.5 mol / L, which is recorded as solution b; the prepared two solutions a and b are slowly added dropwise into the reaction tank in parallel and continuously stirred, and the pH is adjusted with ammonia water so that the pH value is controlled at about 11. Then the resultant was suction-filtered and washed several times in a circulating water vacuum pump until no sulfate ions were detected in the barium chloride solution, and finally it was...
Embodiment 2
[0040] The present embodiment provides lithium-ion battery cathode material LiNi 0.6-x co 0.2 mn 0.2 Al x o 2-y f y , x=0.02, y=0.04; that is, Al and F doping amounts are 0.02 and 0.04 respectively, and nickel sulfate, cobalt sulfate and manganese sulfate are weighed according to the molar ratio Ni:Co:Mn=5.8:2:2, and are prepared into 1.0 mol / L sulfate mixed solution a; mix sodium hydroxide solution and ammonia water to form a mixed solution with a concentration of sodium hydroxide of 2.0 mol / L and a concentration of ammonia water of 0.5 mol / L, which is recorded as solution b; the prepared two solutions a and b are slowly added dropwise into the reaction tank in parallel and continuously stirred, and the pH is adjusted with ammonia water so that the pH value is controlled at about 11. Then the resultant was suction-filtered and washed several times in a circulating water vacuum pump until no sulfate ions were detected in the barium chloride solution, and finally dried and...
Embodiment 3
[0043] The present embodiment provides lithium-ion battery cathode material LiNi 0.6-x co 0.2 mn 0.2 Al x o 2-y f y, x=0.01, y=0.04; that is, Al and F doping amounts are 0.01 and 0.04 respectively, and nickel sulfate, cobalt sulfate, and manganese sulfate are weighed according to the molar ratio Ni:Co:Mn=5.9:2:2, and are formulated to be 1.0 mol / L sulfate mixed solution a; mix sodium hydroxide solution and ammonia water to form a mixed solution with a concentration of sodium hydroxide of 2.0 mol / L and a concentration of ammonia water of 0.5 mol / L, which is recorded as solution b; the prepared two solutions a and b are slowly added dropwise into the reaction tank in parallel and continuously stirred, and the pH is adjusted with ammonia water so that the pH value is controlled at about 11. Then the resultant was suction-filtered and washed several times in a circulating water vacuum pump until no sulfate ions were detected in the barium chloride solution, and finally it was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Initial discharge specific capacity | aaaaa | aaaaa |
| Initial discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com