Anti-tumor nano medicine based on cross-linking biodegradable polymer vesica and preparation method of anti-tumor nano medicine
A technology for degrading polymers and nano-drugs, which is applied in the direction of anti-tumor drugs, drug combinations, and pharmaceutical formulations. Effects of preventing protein denaturation and solving disease problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Synthesis of PEG5k-P(DTC4.4k-TMC19.8k)-bPEI1.8k block copolymer
[0073] The synthesis of PEG5k-P(DTC4.4k-TMC19.8k)-bPEI1.8k is divided into two steps, the first is ring-opening polymerization to prepare PEG5k-P(DTC4.4k-TMC19.8k) diblock copolymer, the specific operation is as follows , in a nitrogen glove box, weigh MeO-PEG-OH ( M n = 5.0 kg / mol, 0.50 g, 100 μmol), TMC (2.0 g, 19.2 mmol) and DTC (0.50 g, 2.60 mmol) were dissolved in dichloromethane (DCM, 7.0 mL), and the ring-opening polymerization catalyst such as Zinc bis(bistrimethylsilyl)amine (29 mg, 75 μmol). The airtight reactor was sealed and placed under magnetic stirring in an oil bath at 40 °C for 2 days. After terminating the reaction with glacial acetic acid, precipitate twice in glacial ether, filter with suction, and dry under vacuum at room temperature to obtain PEG5k-P (DTC4.4k-TMC19.8k).
[0074] Next, PEG5k-P (DTC4.4k-TMC19.8k) was activated by p-nitrophenyl hydroxychloroformate NPC, ...
Embodiment 2
[0076] Example 2 Synthesis of copolymer Mal-PEG6k-P(DTC3.2k-TMC15.4k)-bPEI1.8k
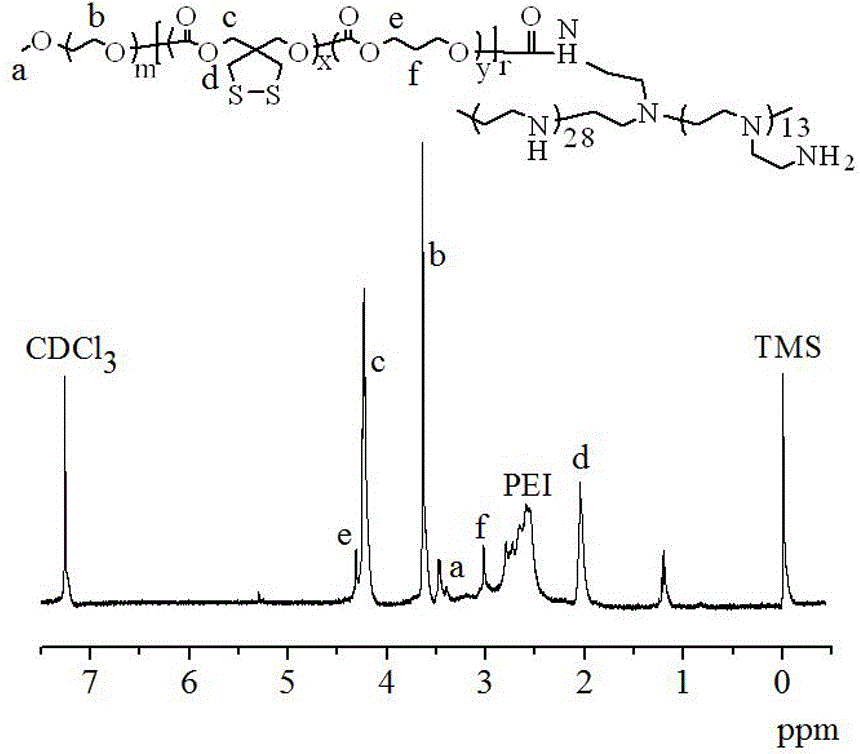
[0077] Its synthesis is similar to Example 1, and it is also divided into two steps, except that the initiator MeO-PEG-OH in the first step is replaced by maleimide-functionalized Mal-PEG6k-OH, and the ring-opening polymerization of TMC and DTC to obtain Mal-PEG6k-P (DTC3.2k-TMC15.4k), and then its terminal hydroxyl was activated by NPC, and then reacted with the primary amine of bPEI1.8k. The specific operation is similar to the first embodiment. Yield: 90.2%. 1 H NMR (400 MHz, DTCl 3 ): PEG: δ 3.38, 3.65; TMC: δ 4.24, 2.05; DTC: δ 4.32, 3.02, PEI: δ 2.56-2.98, and the characteristic peak of Mal. The number-average molecular weight of the polymer was calculated as 6.0-(3.2-15.4)-1.8 kg / mol through the characteristic peak area integral ratio.
[0078]
Embodiment 3
[0079] Example 3 Synthetic polymer Azide-PEG6.5k-P(DTC4.0k-LA15.3)-lPEI0.7k
[0080] Its synthesis is similar to Example 1, and it is also divided into two steps, except that the MeO-PEG-OH in the first step is replaced by azide-functionalized Azide-PEG6.5k-OH, and Azide-PEG6 is obtained by ring-opening polymerization of LA and DTC .5k-P (DTC4.0k-LA15.3), and then its terminal hydroxyl is activated by NPC, and then reacted with the primary amine of linear PEI (lPEI0.7k). Yield: 90.2%. 1H NMR (400MHz, DTCl3): PEG: δ 3.38, 3.65; TMC: δ 4.24, 2.05; DTC: δ 4.32, 3.02, and the characteristic peaks of PEI. The number-average molecular weight of the polymer was calculated as 6.5-(4.0-15.3)-0.7 kg / mol through the characteristic peak area integral ratio.
[0081]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com