Application of Small Molecule Peptides Inhibiting Neuron Apoptosis
A small molecule polypeptide, nerve cell apoptosis technology, applied in the field of biomedicine, to achieve the effects of improving motor function and sensory function, increasing the number of surviving nerve cells, and reducing the number of nerve cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation of embodiment 1 small molecule polypeptide
[0020] The amino acid sequence of the small molecule polypeptide is shown in SEQ ID NO.1. Small molecule peptide synthesis was performed on an ABI 431A solid-phase peptide synthesizer (PE Company, USA). Methods The standard fluorenylmethoxycarbonyl (Fmoc) protocol was used, and arginine was coupled twice. Initially select 0.125mmol p-hydroxymethylphenoxymethyl polystyrene resin (HMP resin), extend the peptide chain from the carboxyl terminal to the amino terminal one by one according to the polypeptide sequence, the amount of each amino acid is 0.5mmol, and the mole of the resin The ratio is 4:1. The α-amino acids of various amino acids are protected by Fmoc, and the other side chain protection groups are: Ser (tBu), Glu (OtBu), Arg (Pmc). The first amino acid is connected to the resin with 4-lutidine, the amino acid is activated with 1-hydroxybenzotriazole and dicycloethyl carbon, and after coupling, the Fm...
Embodiment 2
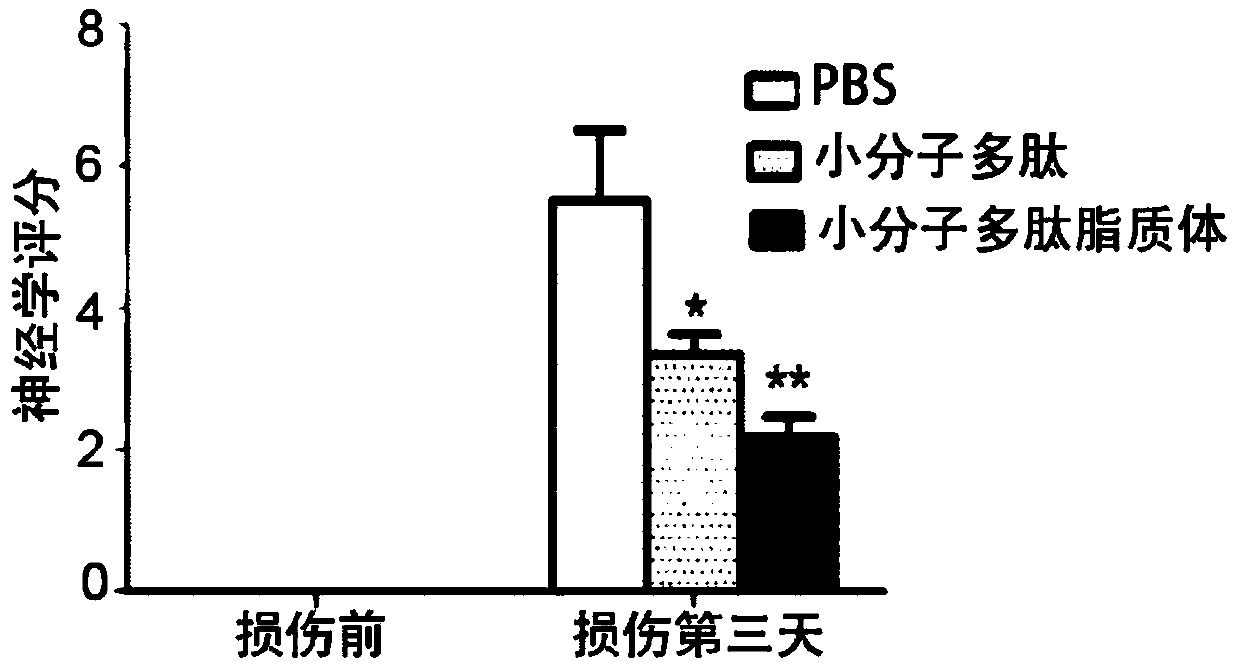
[0022] Example 2 Application of Small Molecular Peptides in Treating Cerebral Hemorrhage in Rats
[0023] 1. Animals: Male SD rats, weighing 250-300 grams, were purchased from the Animal Laboratory of Nantong University, and were normally kept in a clean animal room.
[0024] 2. Drugs: small molecule polypeptide treatment group: intraperitoneal injection of 20 nmol / kg body weight of small molecule polypeptide shown in SEQ ID NO.1, a total of 6 animals; blank group: intraperitoneal injection of the same volume of PBS as the treatment group, There are 6 in total.
[0025] 3. Experimental method: Injecting autologous blood into the brain is a common animal modeling method for cerebral hemorrhage. This experiment is used to verify the therapeutic effect of small polypeptide molecules on cerebral hemorrhage. Inject the autologous blood method in the brain, be specifically: 1. give rat (240-260g) 10% chloral hydrate and carry out intraperitoneal anesthesia (3ml / Kg); Expose the sku...
Embodiment 3
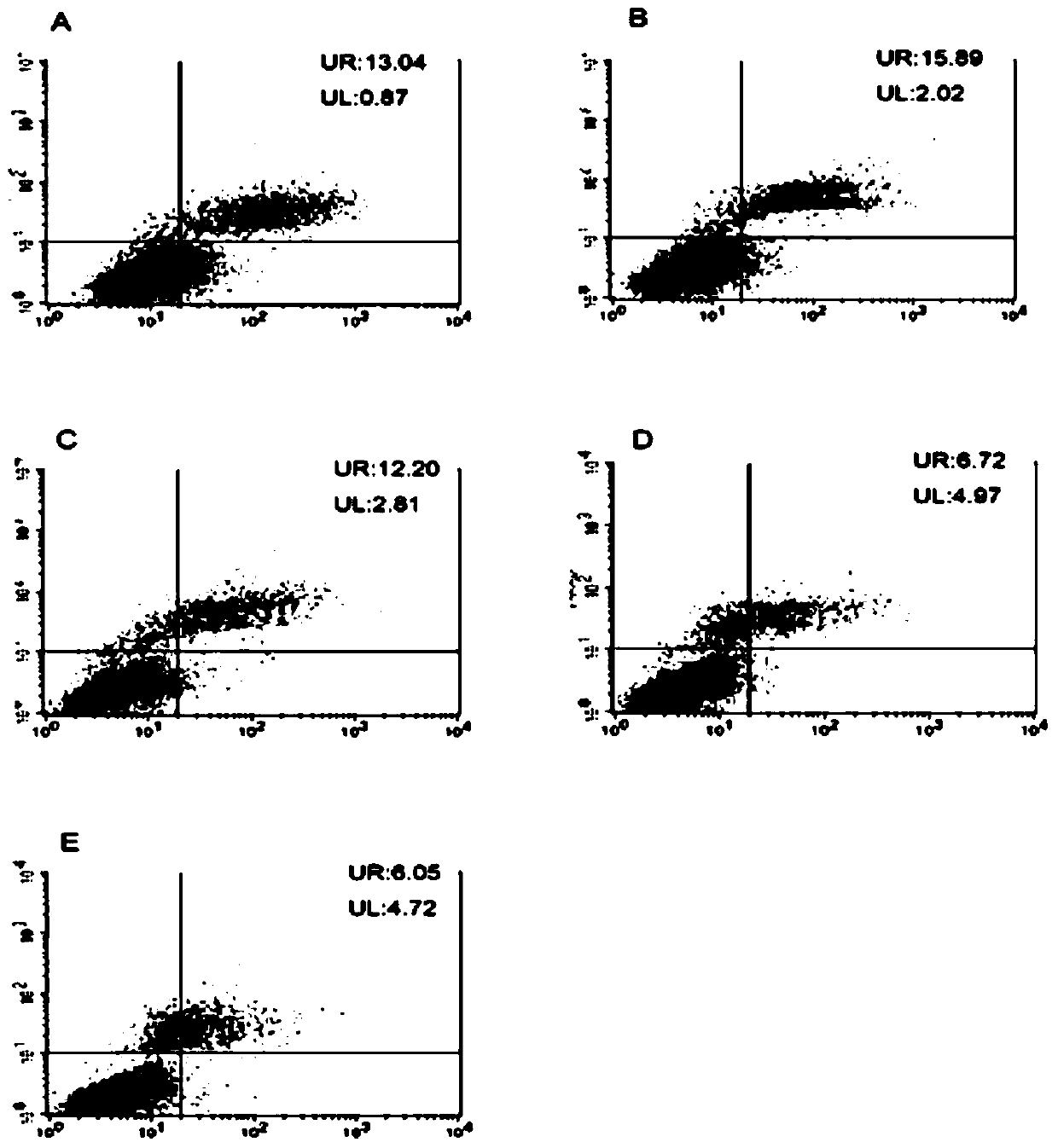
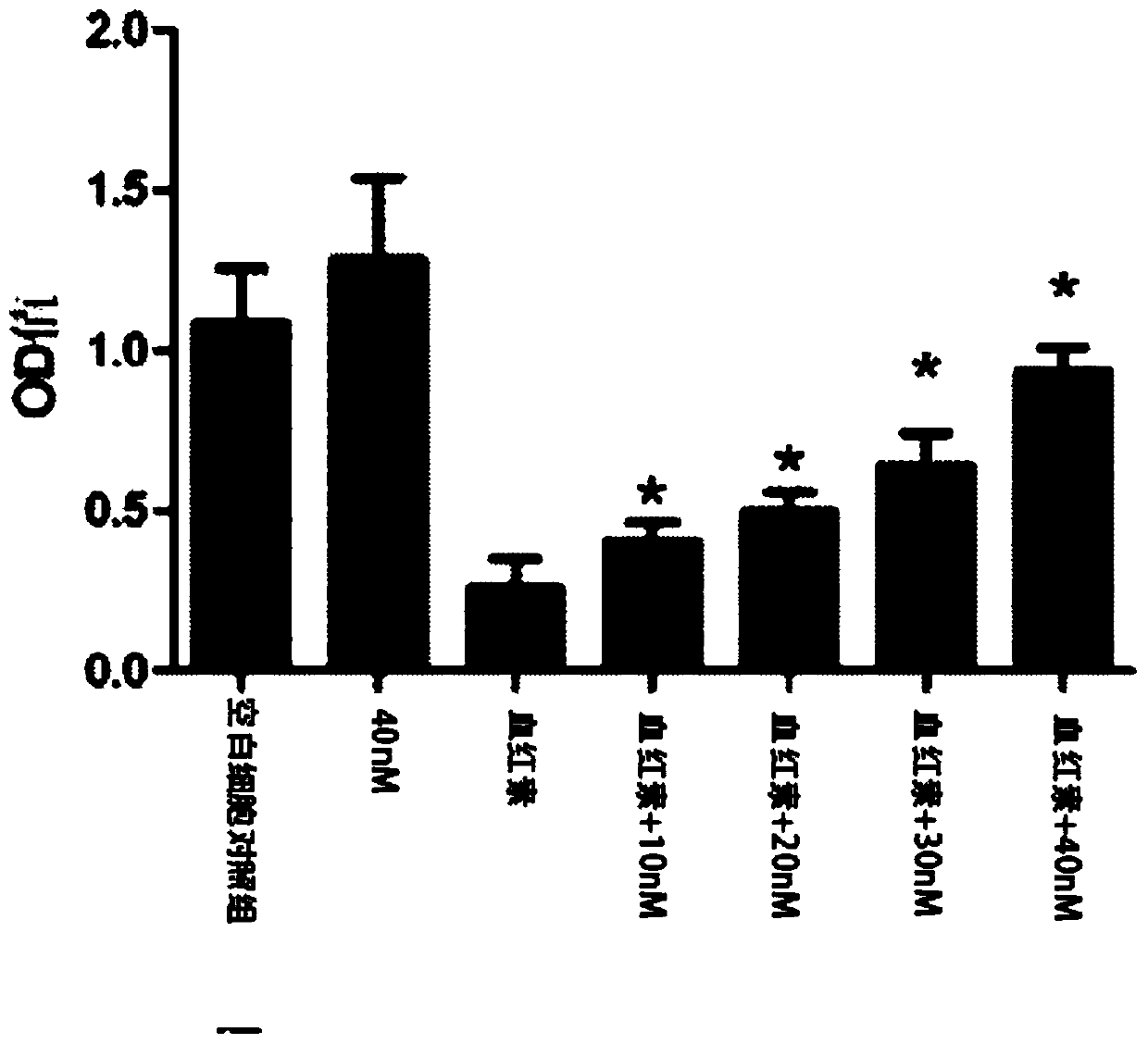
[0040] In order to further utilize in vitro experiments to verify the protection of the present invention to nerve cells and the verification inhibitory effect on macrophages, PC-12 cells (rat adrenal medulla pheochromocytoma cell line) in vitro injury model and LPS-induced macrophage Cellular inflammation model validation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com