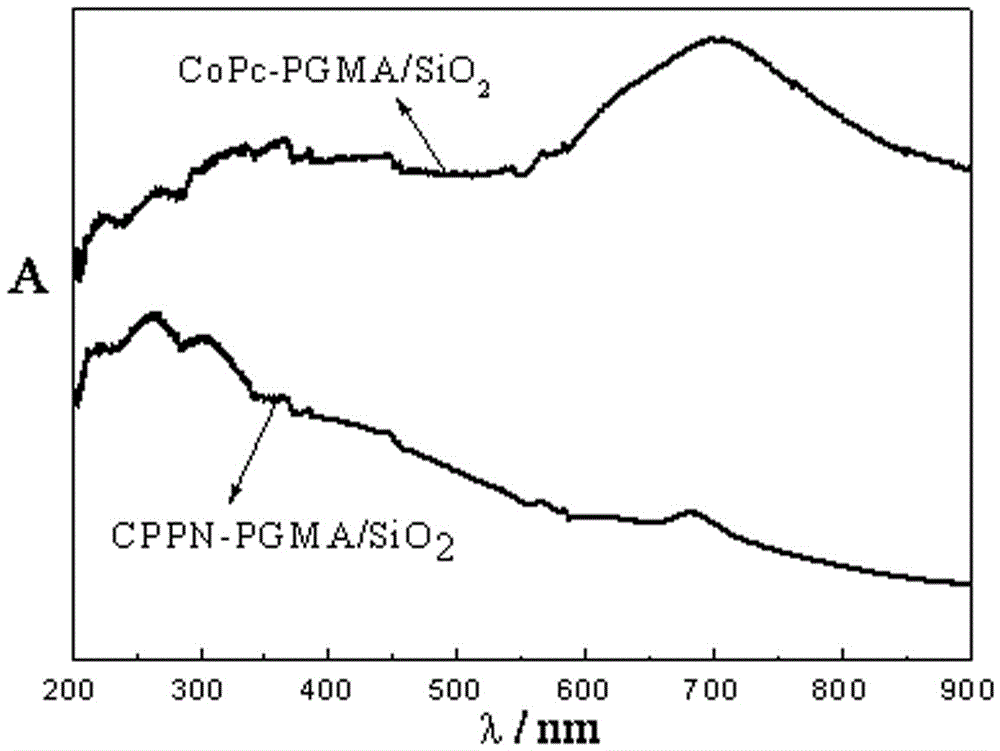
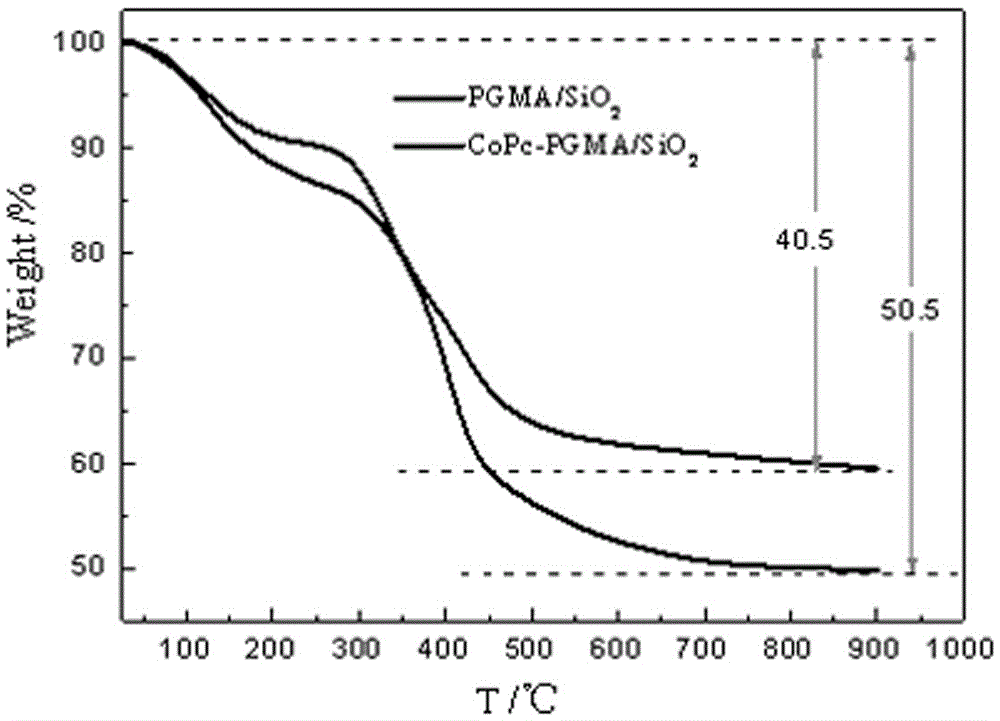
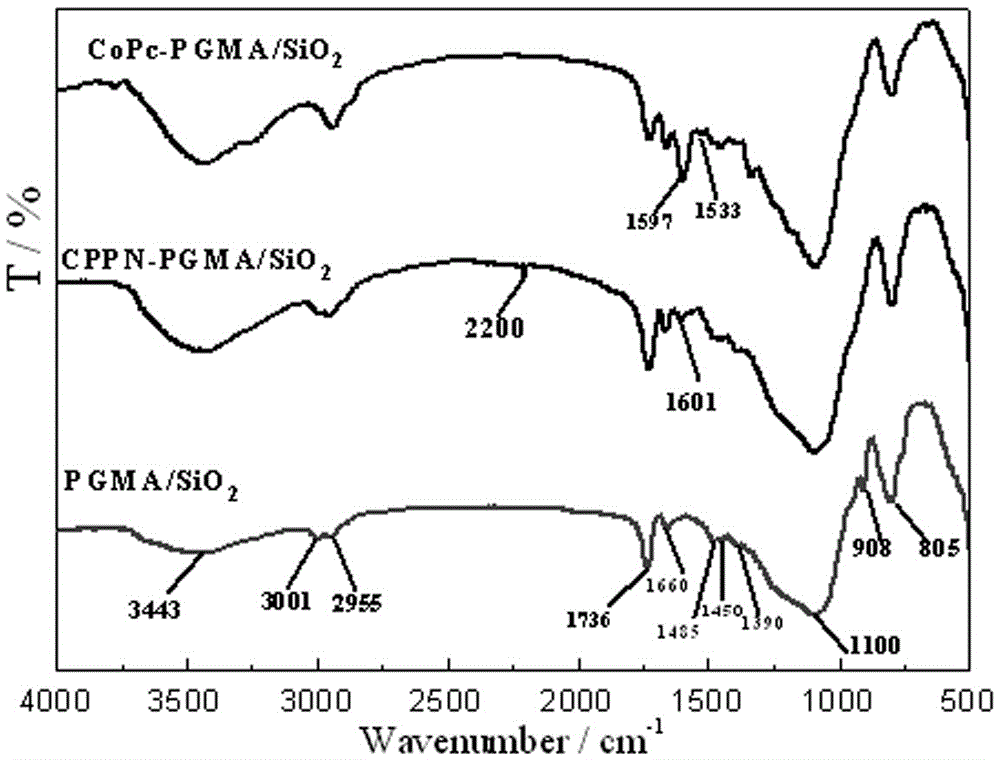
Immobilized metal phthalocyanines catalyst prepared by aid of synchronous synthesis and immobilization processes and methods for preparing and applying immobilized metal phthalocyanines catalyst
A metal phthalocyanine and catalyst technology, which is applied in the field of immobilized metal phthalocyanine catalysts, can solve the problems of difficult preparation process and low immobilization capacity of metal phthalocyanine catalysts, and achieve simple process, low cost and improved thermal stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: In a four-necked flask, add 1.0 g of hybrid material PGMA / SiO 2 and 40 mL of N,N-dimethylformamide, fully soaked and swollen, added 0.30 g of CPPN and 1.5 mL of triethylamine, raised the temperature to 100 ° C under the protection of nitrogen, and reacted at constant temperature for 8 hours, filtered, absolute ethanol and The microspheres were fully washed with distilled water and dried in vacuum to obtain CPPN-PGMA / SiO particles bonded with CPPN 2 , The bonding amount of CPPN is 3.8g / 100g.
[0029] In a four-necked flask, 1.0 g CPPN-PGMA / SiO 2 Soak in 30 mL of n-pentanol to fully swell the particles, then add 0.6 g of 4-nitrophthalonitrile and 0.35 g of cobalt acetate hydrate, and add a certain amount of 2.5 mL of 1, 8-diazacyclo[5, 4, 0] Undec-7-ene, reflux reaction for 12 h under nitrogen protection, cooling and filtering, and repeatedly washing the product particles with methanol, then soaking in concentrated sulfuric acid and washing with distilled wat...
Embodiment 2
[0030] Example 2: In a four-neck flask, add 1.2 g of hybrid material PGMA / SiO 2 and 38 mL of dimethyl sulfoxide, fully soaked and swollen, added 0.35g CPPN and 0.26g NaOH, heated to 90°C under nitrogen protection, reacted at constant temperature for 9h, filtered, fully washed the microspheres with absolute ethanol and distilled water, and dried in vacuum , to get CPPN-PGMA / SiO particles bonded with CPPN 2 , the bonding amount of CPPN is 3.3g / 100g.
[0031] In a four-necked flask, 1.1 g CPPN-PGMA / SiO 2 Soak in 28 mL of n-pentanol to fully swell the particles, then add 0.55 g of 4-nitrophthalonitrile and 0.33 g of manganese acetate hydrate, and add 2.6 mL of 1, 8-diazacyclo[5,4,0 ] Undec-7-ene, reflux reaction under nitrogen protection for 11 h, cooling and filtering, and repeatedly washing the product particles with methanol, then soaking in concentrated sulfuric acid and washing with distilled water to neutrality, and finally using N,N-dimethyl Slightly boil formamide, wash...
Embodiment 3
[0032] Example 3: In a four-necked flask, add 1.1 g of hybrid material PGMA / SiO 2 and 35 mL of dimethyl sulfoxide, after fully soaking and swelling, add 0.32g CPPN and 1.00g Na 2 CO 3 , heated up to 110°C under the protection of nitrogen, reacted at constant temperature for 10 hours, filtered, washed the microspheres with absolute ethanol and distilled water, and dried in vacuum to obtain CPPN-PGMA / SiO particles bonded with CPPN 2 , The bonding amount of CPPN is 3.2g / 100g.
[0033] In a four-neck flask, 1.2 g CPPN-PGMA / SiO 2 Soak in 25 mL of n-pentanol to fully swell the particles, then add 0.5 g of 4-nitrophthalonitrile and 0.21 g of copper acetate hydrate, and add 2.4 mL of 1, 8-diazacyclo[5,4,0 ] Undec-7-ene, reflux reaction for 13 h under the protection of nitrogen, cooled and filtered, and washed the product particles repeatedly with methanol, then soaked in concentrated sulfuric acid and washed with distilled water to neutrality, and finally washed with N,N-dimethyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com