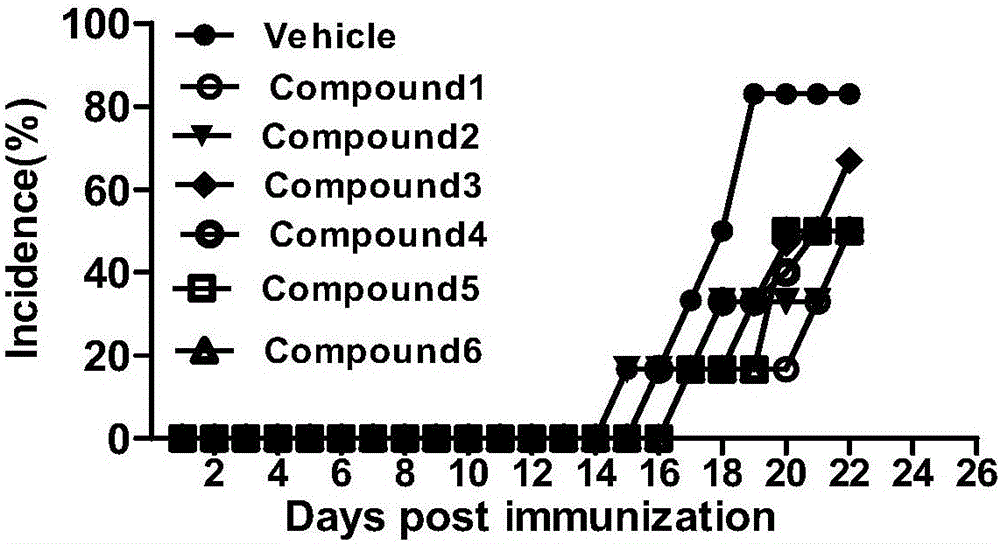
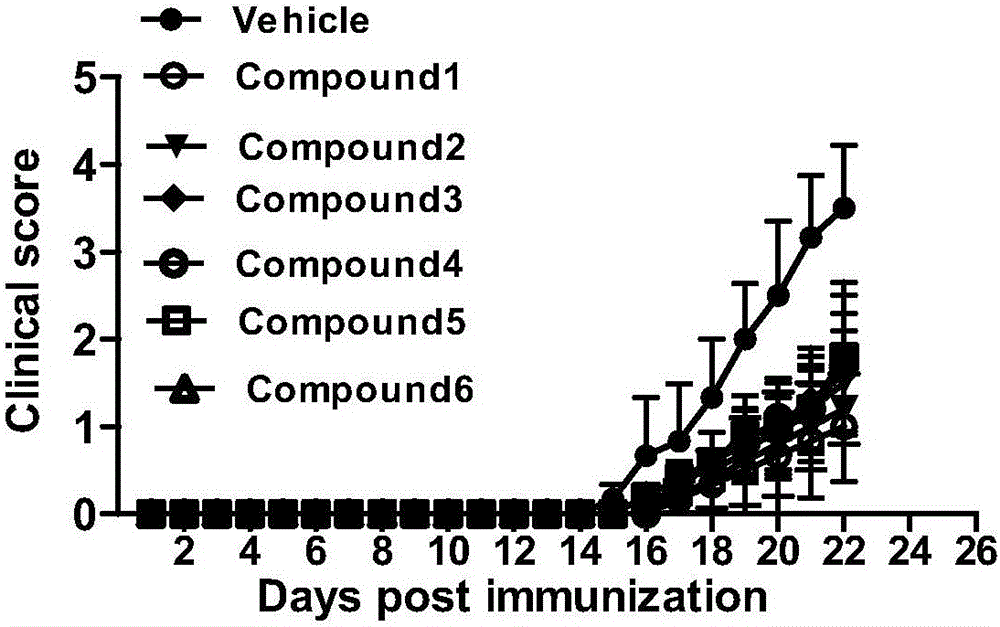
New chalcone derivative, preparation method and application thereof in autoimmune diseases
A chalcone derivative and autoimmune technology, which is applied in the field of medicine, can solve the problems of lack of in-depth evaluation of animal models of autoimmune diseases, limited immune regulation and anti-inflammatory activity, and lack of specificity of immune cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
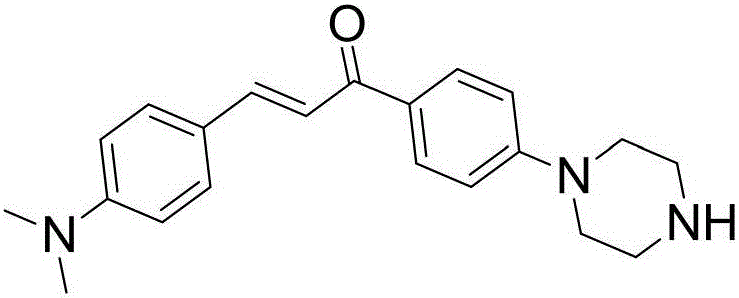
[0019] Embodiment 1: preparation method
[0020] The synthetic method of 4-dimethylamino-4'-(1-piperazinyl)chalcone (the following formula II, referred to as compound 1) is as follows: weigh 4-fluoroacetophenone (2.76g, 20mmol) and p- - Dimethylaminobenzaldehyde (3.28g, 22mmol) In a 100mL round bottom flask, add 30mL ethanol and 20mL 20% KOH, and stir overnight at room temperature. Add 50mL of water, filter with suction, wash the solid twice with 50mL of water and 50mL of 30% ethanol-water successively, and obtain an orange-yellow solid after drying. The above solid and 5.48g (40mmol) potassium carbonate were placed in a 100mL round bottom flask, 5.8g (30mmol) piperazine hexahydrate and 60mL DMF were added, and reacted at 120°C overnight. After cooling, the reactant was poured into 150 mL of water and extracted with DCM (30 mL×3). The organic phase was dried over anhydrous sodium sulfate and concentrated, and the residue was subjected to column chromatography (with 2% CH 3 ...
Embodiment 2
[0044] Example 2: In vitro immune activity screening
[0045] 1. Build a screening model
[0046] Spleen lymphocytes of BALB / C mice undergo a series of changes in morphology and metabolism under the stimulation of mitogen ConA, transform into mother cells, and differentiate and proliferate. In this experiment, 5 μg / ml of ConA (ConA) was added to induce the proliferation of T lymphocytes. Cell proliferation was quantified by MTT assay. Mitochondrial dehydrogenase in living cells can reduce the yellow color of MTT to blue formazan, and the production of formazan is directly proportional to living cells. After dissolving in an organic solvent, the OD value can be detected with a microplate reader.
[0047] Result evaluation: for the proliferation of lymphocytes, the OD value of the tested sample minus the OD value of the control well is used, and the OD value of the control well is multiplied by %.
[0048] A negative sign before the percentage indicates that the sample has a...
Embodiment 3
[0059] Example 3: Anti-inflammatory activity screening in vitro
[0060] 1. Build a screening model
[0061] Raw 264.7 mouse macrophage cell line was purchased from American Cell Bank (ATCC), and cultured in RPMI-1640 medium containing 10% FBS. Raw264.7 cells (1×10 5 Each / well) was inoculated in a 24-well plate, and different concentrations of chalcone derivatives obtained in the present invention were added at the same time, and cultured for 24 hours under the stimulation of 1 μg / ml LPS, and a solvent control and no stimulant background control were set up separately. The culture supernatant was collected by centrifugation (5000rpm, 4°C, 5min), and the NO in the supernatant was detected by the Griess method. 2 - content.
[0062] Table 2. Screening results of anti-inflammatory activity of chalcone derivatives
[0063]
[0064] The results of anti-inflammatory activity screening are shown in Table 2. The six chalcone derivatives significantly inhibited LPS-induced macrop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com