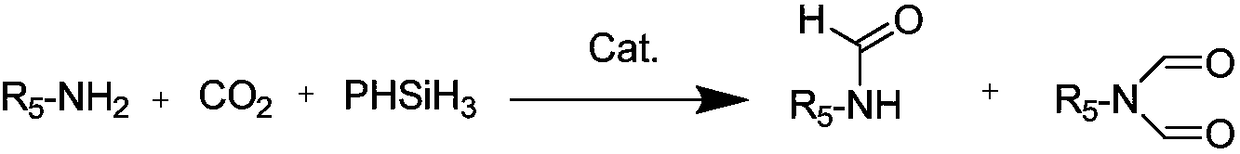
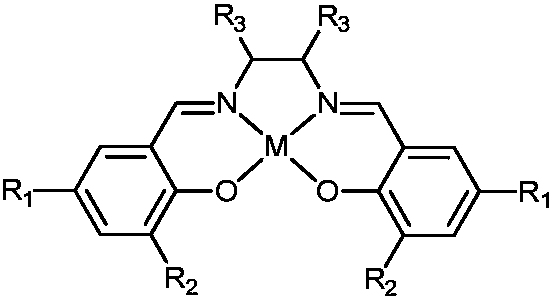
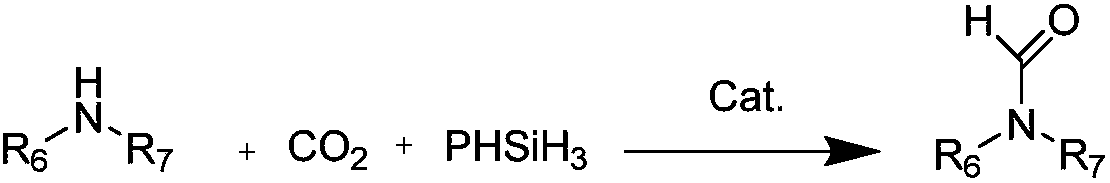A kind of preparation method of n-formamide compounds
A technology for formamides and compounds, which is applied in the field of preparation of N-formamides, can solve the problems of difficult reuse of catalysts, need heavy metal catalysts, complex reaction systems and the like, and achieves low cost, high selectivity and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In the 10mL stainless steel autoclave, add the catalyst of 0.001mmol (M is Zn in the general formula (1), R 1 , R 2 , R 3 are all hydrogen groups) and 0.001mmol cocatalyst (R in general formula (2) 4 is n-butyl), 1mmo N-methylaniline and 1mmol phenylsilane are used as raw materials, the carbon dioxide pressure is adjusted to 0.5MPa, and the stirring reaction is carried out at a temperature of 25°C for 15h. After the reaction is completed, ether is added to centrifuge to recover the catalyst. The solvent was removed by rotary evaporation, and the product N-methylformanilide was obtained after vacuum drying with a yield of 95%.
Embodiment 2
[0037] In the 10mL stainless steel autoclave, add the catalyst of 0.001mmol (M is Al in the general formula (1), R 1 , R 2 , R 3 are all hydrogen groups) and 0.001mmol cocatalyst (R in general formula (2) 4 n-butyl), 1mmo N-methylaniline and 1mmol phenylsilane are used as raw materials, the carbon dioxide pressure is adjusted to 5MPa, and the stirring reaction is carried out at a temperature of 100°C for 3h. After the reaction, ethyl acetate is added to centrifuge to recover the catalyst. The solvent was removed from the clear liquid by rotary evaporation, and the product N-methylformanilide was obtained after vacuum drying with a yield of 99%.
Embodiment 3
[0039] In the 10mL stainless steel autoclave, add the catalyst of 0.001mmol (M is Co in the general formula (1), R 1 and R 3 are all hydrogen groups, R 2 is methyl) and 0.001mmol cocatalyst (R in general formula (2) 4 is n-butyl), 1mmol 2-methoxy-N-methylaniline and 1mmol diphenylsilane are used as raw materials, the pressure of carbon dioxide is adjusted to 1MPa, and the stirring reaction is carried out at a temperature of 60°C for 6h. After the reaction is completed, add two Methane chloride was centrifuged to recover the catalyst, the supernatant liquid was removed by rotary evaporation, and the product 2-methoxy-N-methylformanilide was obtained after vacuum drying with a yield of 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com