Preparation method and application of 2-phenylethanol by non-cell synthetic biology
A technology of synthetic biology and phenylethanol, applied in multi-enzyme systems, fermentation and other directions, to achieve the effects of high catalytic efficiency, simple operation and excellent repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Main reagents: recombinant aromatic aminotransferase I, phenylpyruvate decarboxylase and alcohol dehydrogenase, conventional commercially available or constructed with reference to prior art documents or constructed with reference to the method of Example 5 of the present invention. Chemical standards: L-phenylalanine, oxaloacetic acid, and 2-phenylethanol were purchased from Sigma-Aldrich, USA. All other chemical reagents were domestic analytically pure or chromatographically pure. Main instruments: digital display constant temperature water bath HH-2 (Jiangsu Changzhou Guohua Electric Co., Ltd.), constant temperature metal bath CHB-100 (Jiangsu Hangzhou Bioer Technology Co., Ltd.), precision pH meter PHS-3C (Shanghai Precision Scientific Instrument Co., Ltd. ), Mettler-Toledo electronic balance AB204-N (Shanghai Mettler-Toledo Instrument Co., Ltd.), ultrasonic breaker (Ningbo Science and Technology Co., Ltd.), liquid chromatography (Shanghai Tianmei Company), gas chro...
Embodiment 2
[0059] Multi-enzyme co-reaction synthesis of 2-phenylethanol under the optimal conditions of embodiment 2
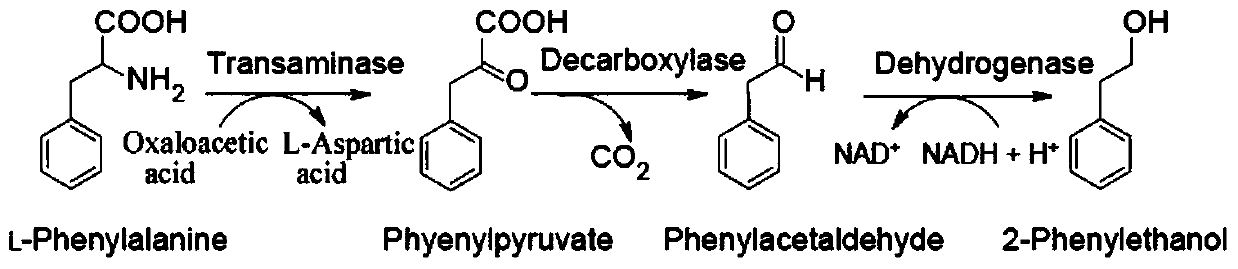
[0060] Refer to Example 1 for the instruments, reagents and detection methods of this embodiment. Using L-phenylalanine and oxaloacetate as substrates, the optimal synthesis of 2-phenylethanol by recombinant aromatic aminotransferase ⅠARO8, phenylpyruvate decarboxylase ARO10 and alcohol dehydrogenase ADH1 was studied. Conditions, all reaction systems were 0.5 mL, containing 0.01 mM pyridoxal phosphate, 0.2 mM thiamine pyrophosphate and 0.1 mM NADPH.
[0061] Using L-phenylalanine and oxaloacetate in a molar ratio of 0.75:l as substrates, 2-phenylethanol was synthesized according to the aforementioned optimal conditions. Recombinases ARO8, ARO10 and ADH1 were mixed at a ratio of 1:0.25:1 according to the activity of the enzymes, and the amount added was 100 μL. 2+ In the reaction system with a concentration of 1.5mM, after 5min in a water bath at 37°C, the reaction was ...
Embodiment 3 3
[0062] Example 3 Co-reaction test in which the three-enzyme co-immobilization system participates
[0063] Main reagents: recombinant aromatic aminotransferase I, phenylpyruvate decarboxylase and alcohol dehydrogenase are commercially available or constructed by referring to conventional techniques in the field, or constructed by referring to the method of Example 5 of the present invention. Chemical standards: L-phenylalanine, oxaloacetic acid, and 2-phenylethanol were purchased from Sigma-Aldrich, USA. Other reagents were routinely purchased from the market, and the above chemical reagents were all domestic analytical or chromatographically pure. Main instruments: digital display constant temperature water bath HH-2 (Jiangsu Changzhou Guohua Electric Appliance Co., Ltd.), electric blast drying oven 101A-3S (Guangdong Guangzhou Fumin Measurement and Control Technology Co., Ltd.), constant temperature metal bath CHB-100 (Jiangsu Hangzhou Bioer Technology Co., Ltd.), Electrolu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com