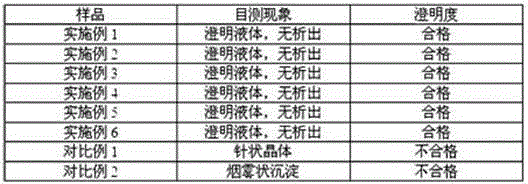
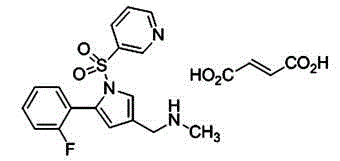
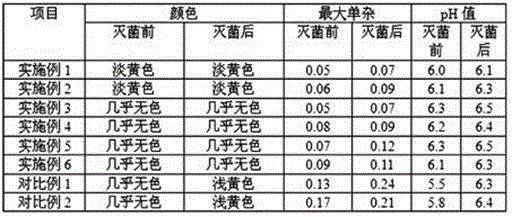
Vonoprazan fumarate injection and preparation method thereof
A technology of flunaprazan fumarate and flunaprazan fumarate, which is applied in the field of pharmaceutical preparations, can solve the effects of restricting and inhibiting gastric acid secretion and treating gastric acid-related diseases, unable to meet swallowing, poor water solubility, etc. problems, to achieve excellent long-term stability, stable product quality, and easy preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 20mg / 20mL each, 1000 vials, the prescription is as follows:
[0056] Flunaprazan fumarate 0.1% (calculated as flunaprazan);
[0057] Alpha Lipoic Acid 0.2%;
[0058] Hydroxypropyl-beta-cyclodextrin 0.5%;
[0059] PEG400 0.2%
[0060] Appropriate amount of phosphate buffer solution, adjust the pH to 6.0-6.5;
[0061] The balance of water for injection.
[0062] Preparation:
[0063] (1) Dissolve hydroxypropyl-β-cyclodextrin in an appropriate amount of ethanol at 40-45°C, add flunaprazan fumarate and lipoic acid and stir to dissolve, filter through a 0.22 μm microporous membrane, add Stir with an appropriate amount of water for injection, concentrate under reduced pressure, add water for injection twice during the concentration process, and remove ethanol under reduced pressure to obtain an aqueous solution of cyclodextrin inclusion, which is set aside;
[0064] (2) Separately take 70% of the prescribed amount of water for injection, add PEG400 and stir to dissolve ...
Embodiment 2
[0068] Example 2 Each 40mg / 20mL, 1000, the prescription is as follows:
[0069] Flunaprazan fumarate 0.2% (calculated as flunaprazan);
[0070] Alpha Lipoic Acid 0.3%;
[0071] Hydroxypropyl-beta-cyclodextrin 0.8%;
[0072] PEG400 0.4%
[0073] Appropriate amount of phosphate buffer solution, adjust the pH to 6.0-6.5;
[0074] The balance of water for injection.
[0075] Preparation:
[0076] (1) Dissolve hydroxypropyl-β-cyclodextrin in an appropriate amount of ethanol at 40-45°C, add flunaprazan fumarate and lipoic acid and stir to dissolve, filter through a 0.22 μm microporous membrane, add Stir with an appropriate amount of water for injection, concentrate under reduced pressure, add water for injection twice during the concentration process, and remove the solvent under reduced pressure to obtain an aqueous solution of cyclodextrin inclusion, which is set aside;
[0077] (2) Separately take 70% of the prescribed amount of water for injection, add PEG400 and stir to d...
Embodiment 3
[0081] Example 3 Each 20mg / 20mL, 1000, the prescription is as follows:
[0082] Flunaprazan fumarate 0.1% (calculated as flunaprazan);
[0083] Lysine 0.2%;
[0084] Sulfobutyl ether-4-beta-cyclodextrin 0.6%;
[0085] PVP-K30 0.3%;
[0086] Appropriate amount of phosphate buffer solution, adjust the pH to 6.0-6.5;
[0087] The balance of water for injection.
[0088] Preparation:
[0089] (1) Dissolve sulfobutyl ether-4-β-cyclodextrin in an appropriate amount of ethanol at 40-45°C, add flunaprazan fumarate and lysine and stir to dissolve, filter through 0.22 μm microporous Membrane filtration, add water for injection and stir, concentrate under reduced pressure, add water for injection twice during the concentration process, remove ethanol under reduced pressure, obtain cyclodextrin inclusion aqueous solution, and set aside;
[0090] (2) Separately take 70% of the prescribed amount of water for injection, add PVP-K30 and stir to dissolve to obtain a clear liquid;
[009...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com