Cyanohydrins lyase and preparing method and application thereof
A lyase and cyanohydrin technology, applied in the field of cyanohydrin lyase, can solve the problems of insufficient enzyme activity, difficult to reach, low soluble protein content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0102] The preparation method of S-cyanohydrin
[0103] The present invention also provides a kind of preparation method of S-cyanohydrin, described method comprises steps:
[0104] (1) contacting the mutant cyanohydrin lyase of the present invention with a reaction substrate to carry out a catalytic reaction, thereby generating the S-cyanohydrin;
[0105] (2) Separating and purifying the S-cyanohydrin product.
[0106] In a preferred embodiment of the present invention, in the step (1), the reaction substrate is m-phenoxybenzaldehyde and acetone cyanohydrin (or, hydrogen cyanide).
[0107] In a preferred embodiment of the present invention, in the step (1), the temperature of the catalytic reaction is 0-20°C.
[0108] The main advantages of the present invention are:
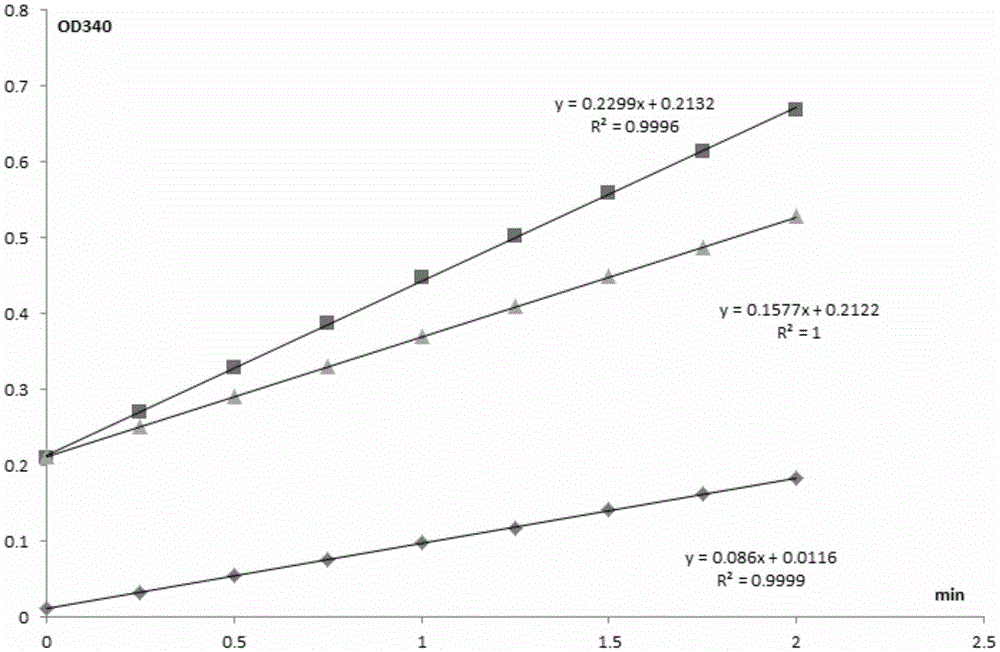
[0109] (1) The mutated cyanohydrin lyase of the present invention has catalytic activity much higher than that of the wild type cyanohydrin lyase, which is about twice that of the wild type;
[0110] (2) Th...
Embodiment 1
[0113] Construction of embodiment 1 mutant
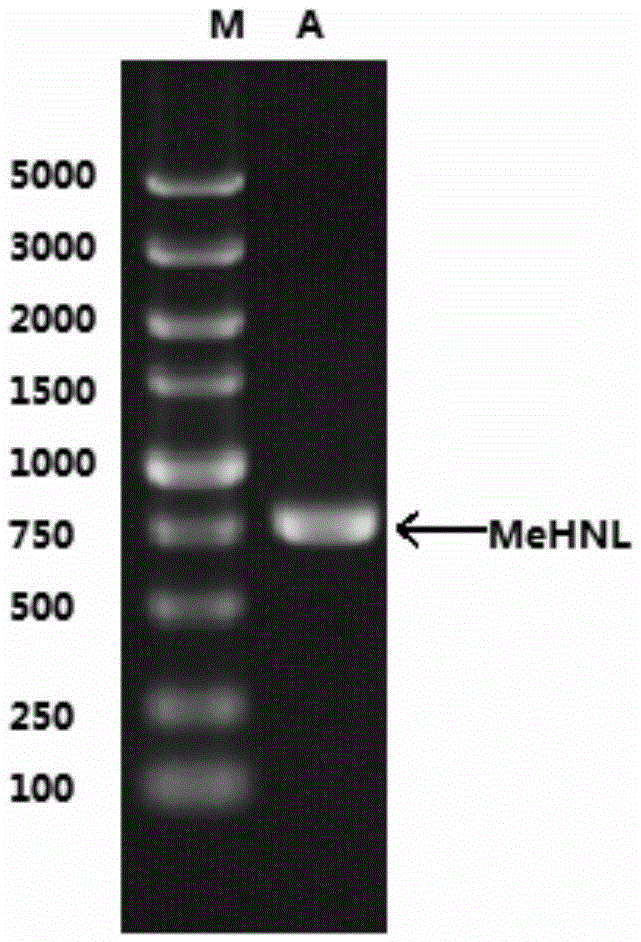
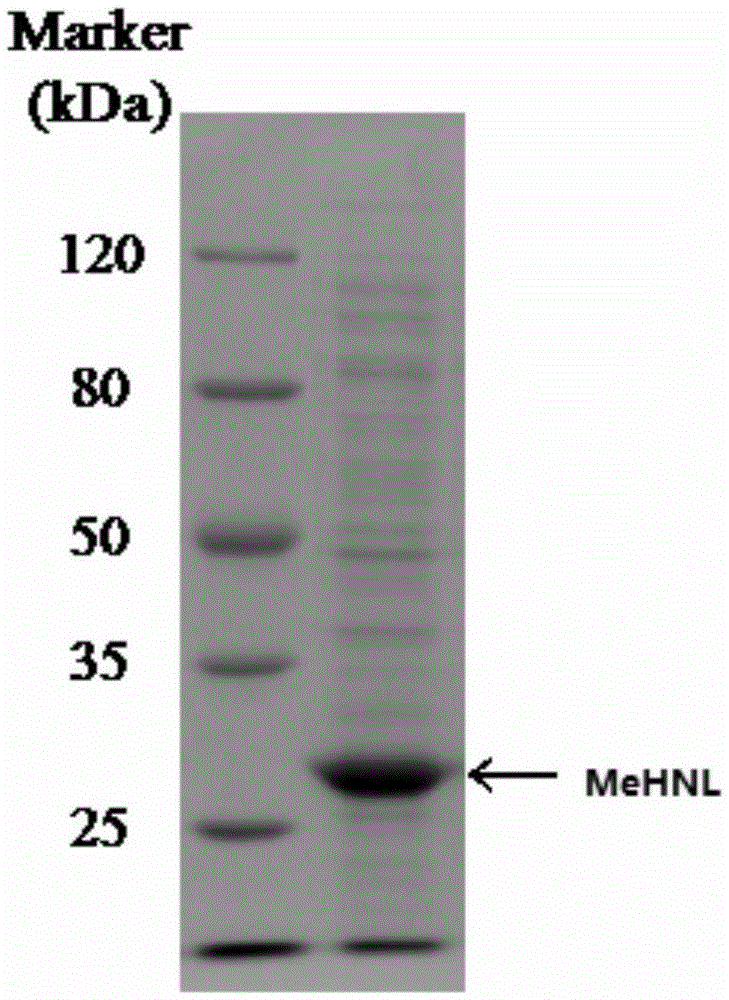
[0114] According to the protein sequence 1 (SEQ ID NO.: 1) of MeHNL reported by Wajant, according to the codon preference of Escherichia coli, MeHNL DNA sequence 2 (SEQ ID NO.: 2) was synthesized and cloned into plasmid pET28 (purchased from Invitrogene company) NdeI-HindIII site. Using the plasmid as a template, primers were designed, see sequence 3 and sequence 4. Using a random mutagenesis kit, by changing the MnSO 4 concentration to carry out multiple reactions, thereby introducing 1 to 3 point mutations into the MeHNL gene, so that 1-3 amino acids in the amino acid sequence of the MeHNL enzyme are replaced. To amplify the enzyme encoding MeHNL, primers SEQ ID No.3 and SEQ ID No.4 were used as forward and reverse primers, respectively. Both primers contained sites compatible with the PCR-amplified MeHNL gene fragment obtained by site-directed recombination cloning using Gateway Technology. The gel electrophoresis profile of ...
Embodiment 2
[0123] Example 2 strain construction and high-density fermentation
[0124] Synthesize a tryptophan promoter containing sequence 6 (SEQ ID NO.: 6), and connect it to the NcoI and NdeI sites of pET28a, and then connect the coding polynucleotides of sequence 1 and 5 in Example 1 to the aforementioned The NdeI-XhoI site of the Escherichia coli plasmid pTrp2-MeHNL1 and pTrp2-MeHNL5 containing the tandem tryptophan promoter (plasmid map as shown in Figure 4 shown). The plasmid was transformed into Escherichia coli JM109 (purchased from Invitrogene), and the corresponding strains were obtained on Kana resistance plates, inoculated into LB medium, cultured overnight at 37°C, and preserved with 20% glycerol.
[0125] The strains were inoculated in a 1L shake flask filled with 200mL LB medium, and cultured at 37°C, 180-220rpm for 10-16h. The above-mentioned cultivated seeds were inoculated in a 3L upper tank fermentation medium (M9) in a ratio of 10% (v / v) (glucose 4g / L, disodium hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com