Microfluid preparation method for tumor therapeutic vaccine nanocarriers
A technology of nanoparticles and carrier materials, which is applied in the direction of antineoplastic drugs, chemical instruments and methods, and medical preparations of non-active ingredients. It can solve the problems of insufficient uniformity of nanoparticles, poor controllability and repeatability of experiments, etc. Simple, reproducible, and biologically effective results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] Preparation method of nanoparticles loaded with negatively charged proteins
[0061] The preparation method of the nanoparticle loaded with negatively charged protein of the present invention comprises the steps of:
[0062] (1) dissolving the negatively charged protein to obtain a protein solution;
[0063] (2) dissolving the carrier material to obtain a carrier material solution;
[0064] (3) The protein solution and the carrier material solution are pumped into the Tesla structure chip from different channels, and mixed slightly to obtain nanoparticles loaded with negatively charged proteins.
[0065] The negatively charged protein may be an antigenic protein.
[0066] In one embodiment, the negatively charged protein is OVA. In another embodiment, the negatively charged protein is BSA. However, the negatively charged protein is not limited to specific OVA or BSA, as long as the protein is negatively charged. OVA, the full name Ovalbumin, refers to chicken ovalb...
Embodiment 1
[0099] Embodiment 1 adopts Tesla structure chip to prepare PEI-OVA nanoparticle
[0100] (1) First, dissolve the negatively charged protein OVA in a buffer solution of 1 mM HEPES, pH=7.4, and prepare a 10 mg / ml OVA solution;
[0101] (2) dissolving branched PEI with a molecular weight of 25KDa in deionized water to obtain a PEI solution of 0.125 mg / ml to 3.0 mg / ml;
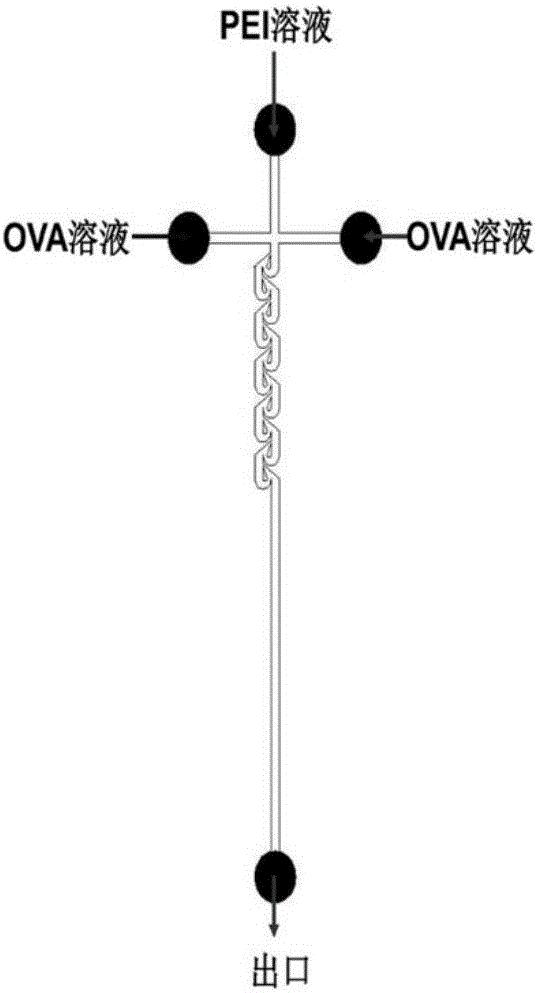
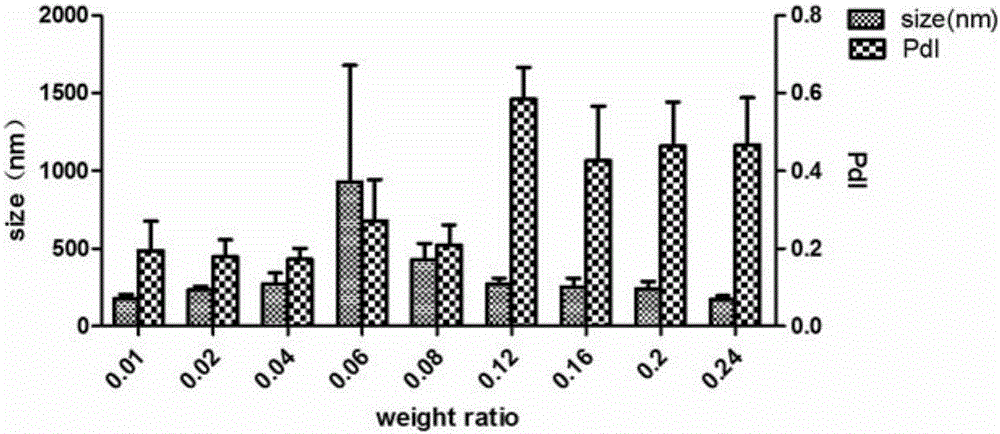
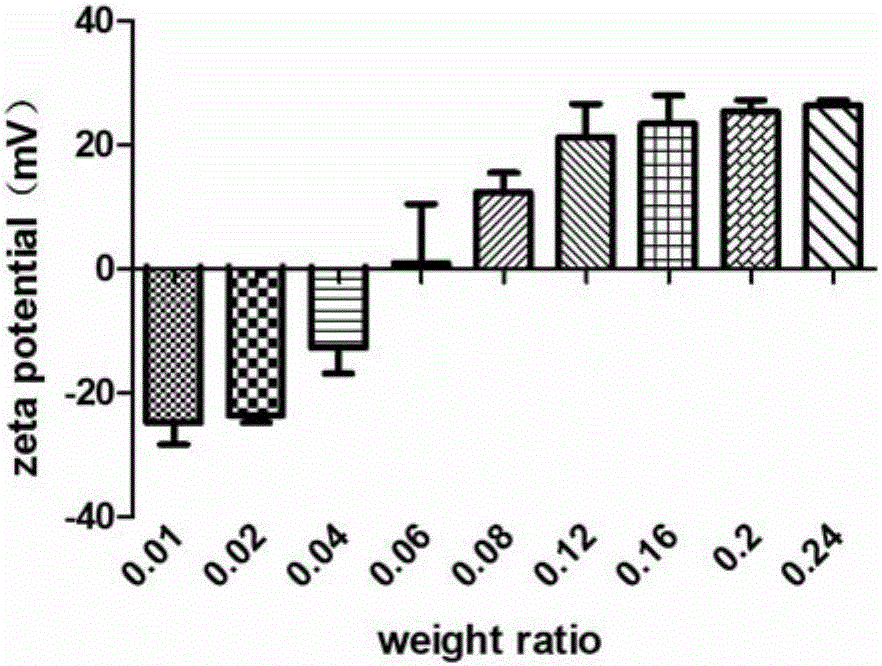
[0102] (3) The OVA solution obtained in step (1) is divided into two 1ml syringes, the PEI solution obtained in step (2) is also added to the 1ml syringe, the air bubbles in the syringe are removed, and the pathway is connected. The OVA solution and the PEI solution were simultaneously pumped into the Tesla structure chip for micro-mixing (perfusion method such as figure 1 As shown), the composite solution of PEI and OVA is obtained, the mass ratio range is 0.01~0.24, wherein the width of the path of the Tesla structure chip is 200 μm, the height is 110 μm, the perfusion volume of the PEI solution channel is 100 ...
Embodiment 2
[0109] Example 2 Study on the Biological Effects of PEI-OVA Nanoparticles Prepared by Tesla Structure Chip
[0110] PEI-OVA nanoparticles were prepared by referring to the method of Example 1, and the mass ratio of PEI to OVA ranged from 0.01 to 0.24. Dilute the prepared PEI-OVA nanoparticle solutions with different mass ratios to an OVA concentration of 0.5 mg / ml, take 30 μl of the PEI-OVA nanoparticle dilution and add it to a 96-well plate with 120 μl of dendritic cell solution, and the cell density is 8×10 5 / ml, co-incubated overnight in a 37°C cell culture incubator, then added 120 μl of B3Z cell solution into the well, and incubated together for 24 hours, and finally detected the concentration of IL-2 secreted by B3Z cells with an ELISA kit to investigate PEI-OVA nano Antigen presentation effect of particles.
[0111] Experimental group: 1640+FBS group, refers to the addition of 1640 and FBS to the medium of dendritic cells.
[0112] Conclusion: Results Figure 4 As ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com