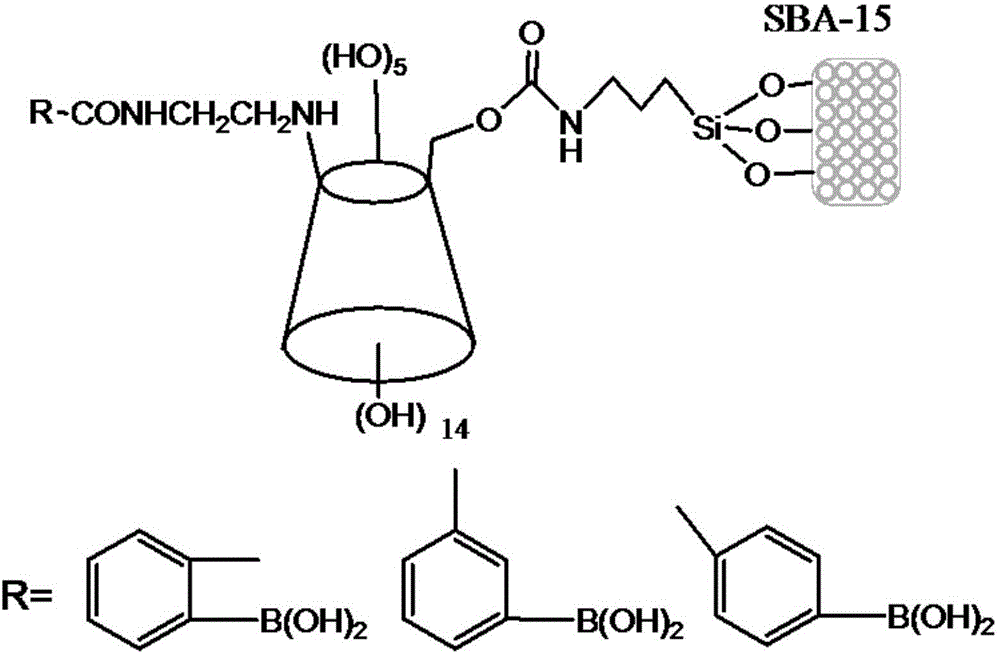
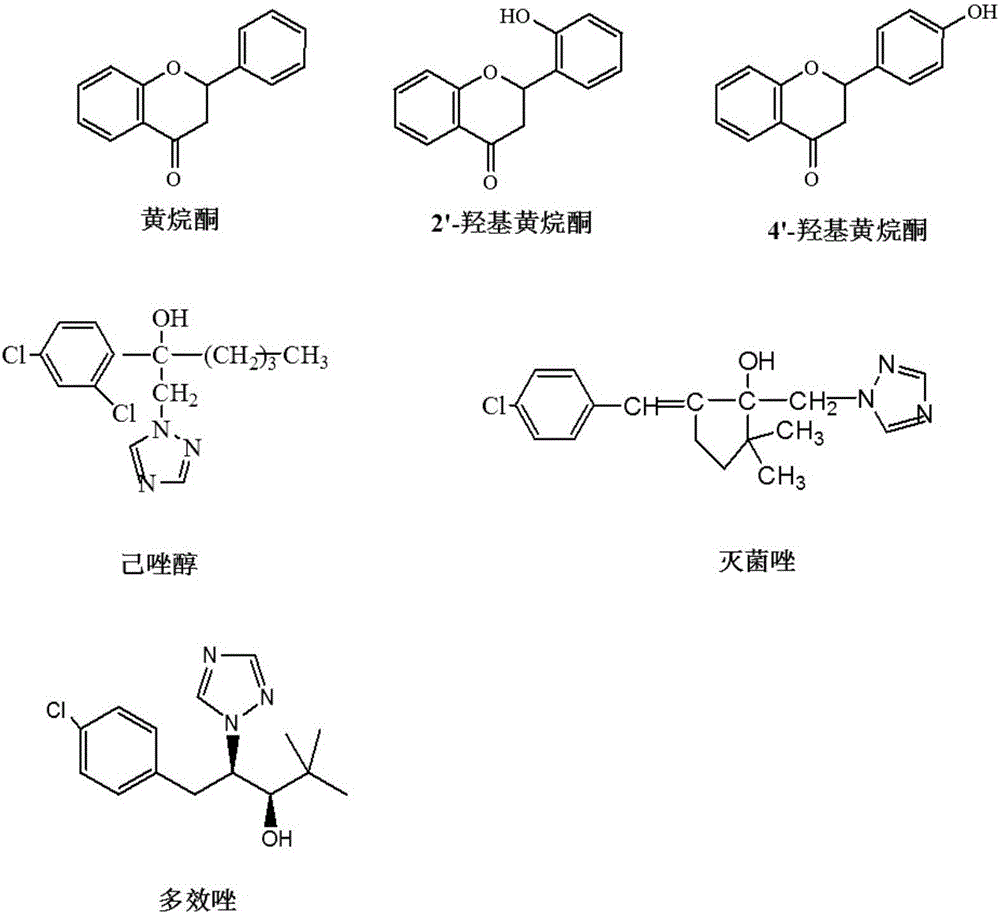
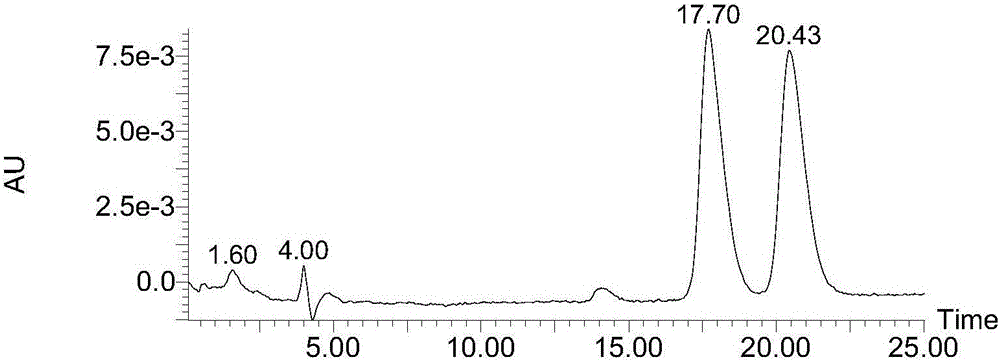
Borono benzoylated beta-cyclodextrin bonded silica gel and uses thereof
A technology of boronic acid benzoyl and acid benzoyl, which is applied in the field of preparation of bonded SBA-15 silica gel chiral stationary phase, and can solve the problems of poor chiral separation performance of chiral stationary phase materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] A kind of boronic acid group benzoylation β-cyclodextrin bonded silica gel, is prepared by following method:
[0072]1) Under a nitrogen atmosphere, use anhydrous dimethyl sulfoxide as a solvent, and use the mmol dosage of ethylenediamine β-cyclodextrin as the raw material: the mmol dosage of (o-, m-, p-)-carboxyphenylboronic acid: dimethyl The ratio of the amount of sulfoxide in ml is 1.0:0.6~1.0:40~50, add 2.0~4.0mmol N-hydroxysuccinimide, 2.0~3.0mmol1-ethyl-3-(3-dimethylaminopropyl ) carbodiimide hydrochloride, stirring for 1-2 hours to fully dissolve the raw materials to form a homogeneous solution; in an oil bath at 35-40 ° C, magnetically stirred for 18-20 hours; then filtered and mixed with dimethyl sulfoxide, water, After washing with methanol and acetone in sequence, and drying in vacuum at 50°C, the boronic acid-benzoylated β-cyclodextrin ligand is obtained;
[0073] 2) Under a nitrogen atmosphere, dissolve the borate-benzoylated β-cyclodextrin obtained in st...
Embodiment 2
[0087] Take SBA-15 (400m 2 / g) Activated silica gel 2.5g as the base.
[0088] 1) Under a nitrogen atmosphere, use anhydrous dimethyl sulfoxide as a solvent, and prepare the raw materials according to the mmol amount of ethylenediamine β-cyclodextrin: the mmol amount of o-carboxyphenylboronic acid: the ml amount of dimethyl sulfoxide Add the ratio of 1.0:0.6:40 to the round bottom flask, add 2.0mmol N-hydroxysuccinimide, 2.0mmol 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride Salt, stir for 1 hour to fully dissolve the raw materials to form a homogeneous solution; configure a condenser tube and a calcium chloride drying tube, and react with magnetic stirring for 18 hours in an oil bath at 35°C; then filter and mix with dimethyl sulfoxide, water, methanol, After washing with acetone in sequence and vacuum drying at 50°C, o-boronic acid benzoylated β-cyclodextrin was obtained, and the reaction yield of this step was 55%;
[0089] 2) Under a nitrogen atmosphere, dis...
Embodiment 3
[0096] Take SBA-15 (500m 2 / g) Activated silica gel 2.5g as the base.
[0097] 1) Under a nitrogen atmosphere, use anhydrous dimethyl sulfoxide as a solvent, and prepare the raw materials according to the mmol amount of ethylenediamine β-cyclodextrin: the mmol amount of o-carboxyphenylboronic acid: the ml amount of dimethyl sulfoxide Add the ratio of 1.0:1.0:50 to the round bottom flask, add 4.0mmol N-hydroxysuccinimide, 3.0mmol 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride Salt, stir for 2 hours to fully dissolve the raw materials to form a homogeneous solution; configure a condenser tube and a calcium chloride drying tube, and react with magnetic stirring for 20 hours in a 40°C oil bath. Then, after filtering, washing with dimethyl sulfoxide, water, methanol and acetone in sequence, and vacuum-drying at 50°C, the o-boronic acid benzoylated β-cyclodextrin ligand was obtained, and the reaction yield of this step was 67%;
[0098] 2) Under a nitrogen atmosphere,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com