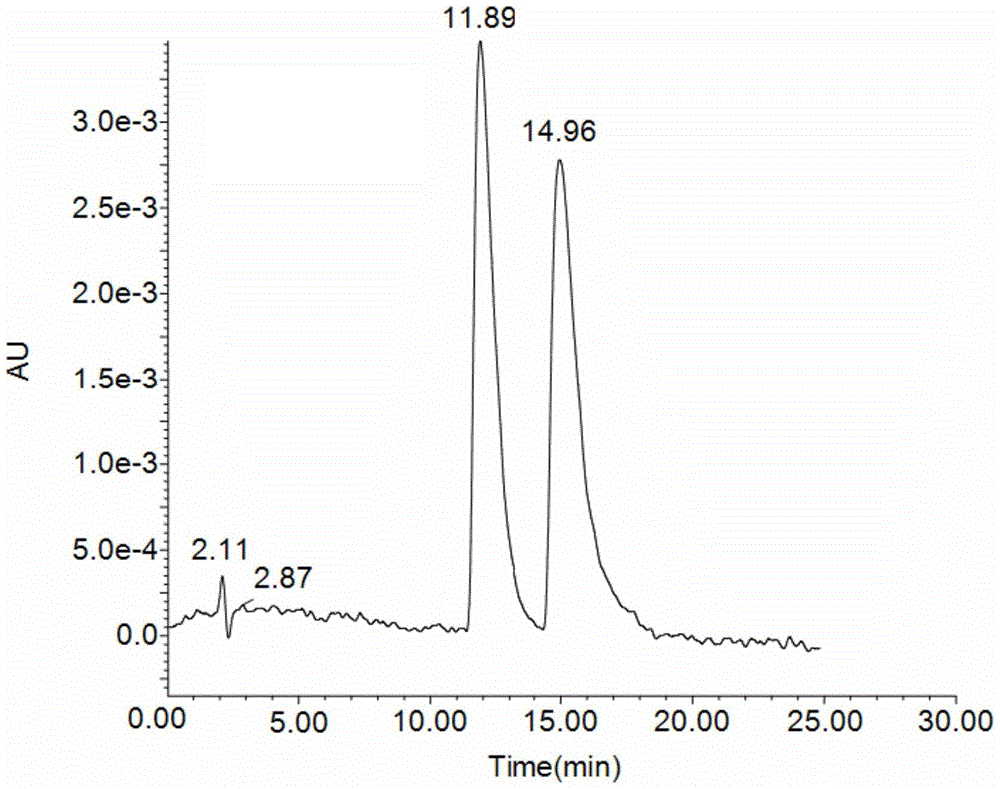
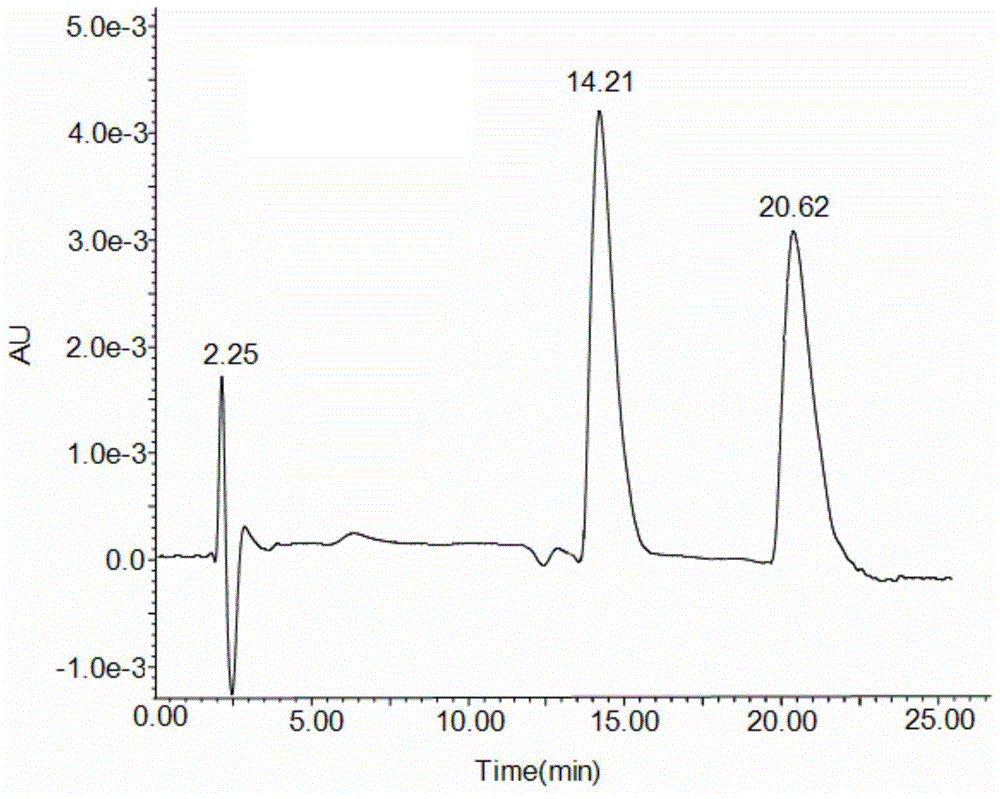
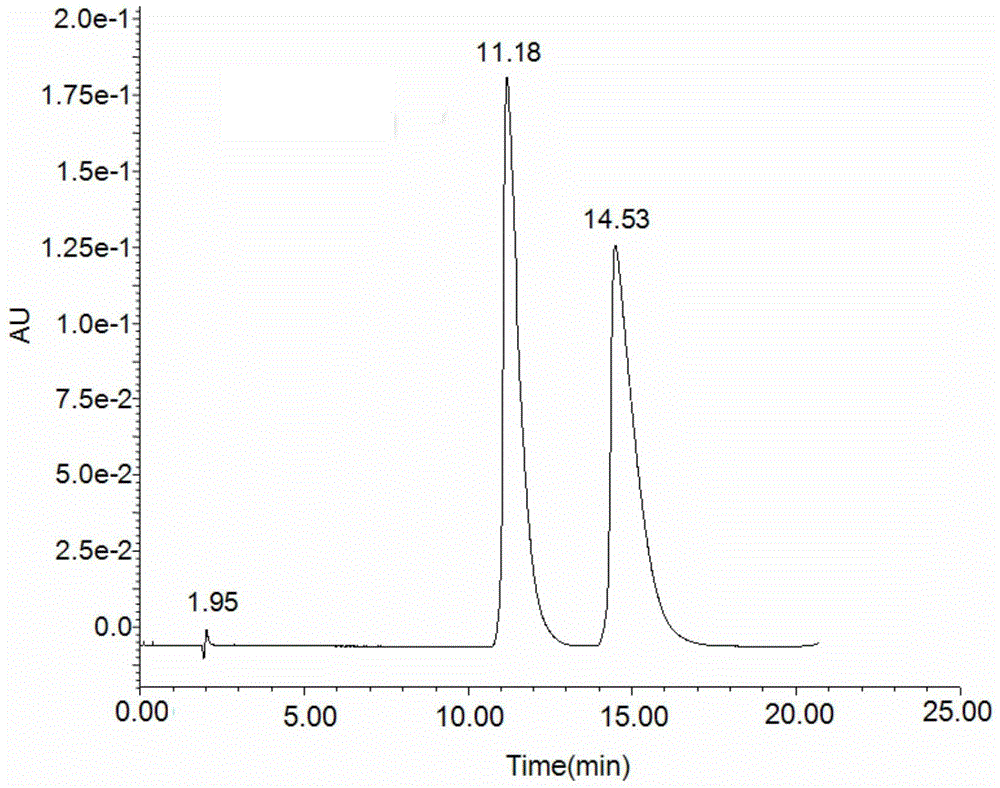
Preparation method and application of 6-benzylphenethylamine derivatized β-cyclodextrin bonded SBA-15
A technology of benzylphenethylamine and cyclodextrin is applied in the field of preparation of chiral stationary phases, which can solve the problems of affecting chiral separation effect, inter-batch chromatographic separation performance of difficult chiral stationary phases, and cyclodextrin port congestion. , to improve the chiral separation ability and range, improve the chiral recognition effect, and reduce the mass transfer resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take SBA-15 (400m 2 / g) Activated silica gel 2.5g as the base.
[0034] 1) Under a nitrogen atmosphere, use anhydrous N,N-dimethylformamide as a solvent to sulfonylate β-cyclodextrin (mmol) with 6-p-toluene: N-benzyl-1-phenylethylamine ( ml) at a ratio of 1.0:0.6, add 6-p-toluenesulfonylated β-cyclodextrin and N-benzyl-1-phenylethylamine into a round bottom flask, stir for 1 hour to fully dissolve the raw materials to form a homogeneous solution ; Configure a condenser tube and a calcium chloride drying tube, under the protection of nitrogen, in an 80 ° C oil bath, magnetically stir the reaction for 4 hours; then evaporate the solvent under reduced pressure, dissolve the solid in a small amount of hot water, and add acetone- Aqueous solution (10:1, v / v), collect the white precipitate, and repeat the above operation 3 times, after drying in a vacuum oven at 50°C, 6-benzylphenethylamine β-cyclodextrin is obtained, and the reaction yield of this step is 60%;
[0035] 2)...
Embodiment 2
[0042] Take SBA-15 (500m 2 / g) Activated silica gel 2.5g as the base
[0043] 1) Under a nitrogen atmosphere, using anhydrous N,N-dimethylformamide as a solvent, according to 6-p-toluenesulfonylation of β-cyclodextrin (mmol): N-benzyl-1-phenylethylamine ( ml) at a ratio of 1.0:1.0, add 6-p-toluenesulfonylated β-cyclodextrin and N-benzyl-1-phenylethylamine into a round bottom flask, stir for 2 hours to fully dissolve the raw materials to form a homogeneous solution ; configure a condenser tube and a calcium chloride drying tube, under the protection of nitrogen, in an 85 ° C oil bath, magnetically stir the reaction for 6 hours; then evaporate the solvent under reduced pressure, dissolve the solid in a small amount of hot water, and add acetone- aqueous solution (10:1, v / v), collect the white precipitate, and repeat the above operation 3 times, after drying in a vacuum oven at 50°C, 6-benzylphenethylamine β-cyclodextrin is obtained, and the reaction yield of this step is 68%; ...
Embodiment 3
[0051] Take SBA-15 (420m 2 / g) Activated silica gel 2.5g as the base.
[0052] 1) Under a nitrogen atmosphere, using anhydrous N,N-dimethylformamide as a solvent, according to 6-p-toluenesulfonylation of β-cyclodextrin (mmol): N-benzyl-1-phenylethylamine ( ml) at a ratio of 1.0:0.8, add 6-p-toluenesulfonylated β-cyclodextrin and N-benzyl-1-phenylethylamine into a round bottom flask, stir for 1.5h to fully dissolve the raw materials to form a homogeneous phase solution; equipped with a condenser tube and a calcium chloride drying tube, under the protection of nitrogen, in an oil bath at 85°C, and reacted with magnetic stirring for 5 hours. Then evaporate the solvent under reduced pressure, dissolve the solid in a small amount of hot water, add acetone-water solution (10:1, v / v) with stirring, collect the white precipitate, and repeat the above operation 3 times, and dry in a vacuum oven at 50°C Finally, 6-benzylphenethylamine β-cyclodextrin was obtained, and the reaction yiel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com