Preparation method of crosslinking halohydrin dehalogenase aggregate
A halohydrin dehalogenase, aggregate technology, applied in biochemical equipment and methods, halocarbon lyase, immobilized on/in organic carriers, etc. possible, low fixing efficiency, etc., to achieve the effect of high reuse rate, low cost, and high mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A method for preparing cross-linked halohydrin dehalogenase aggregates, comprising the following steps:
[0041] Step A: Prepare the crude enzyme solution for halohydrin dehalogenation; specifically, the crude enzyme solution is prepared by constructing recombinant Escherichia coli, and the steps are as follows:
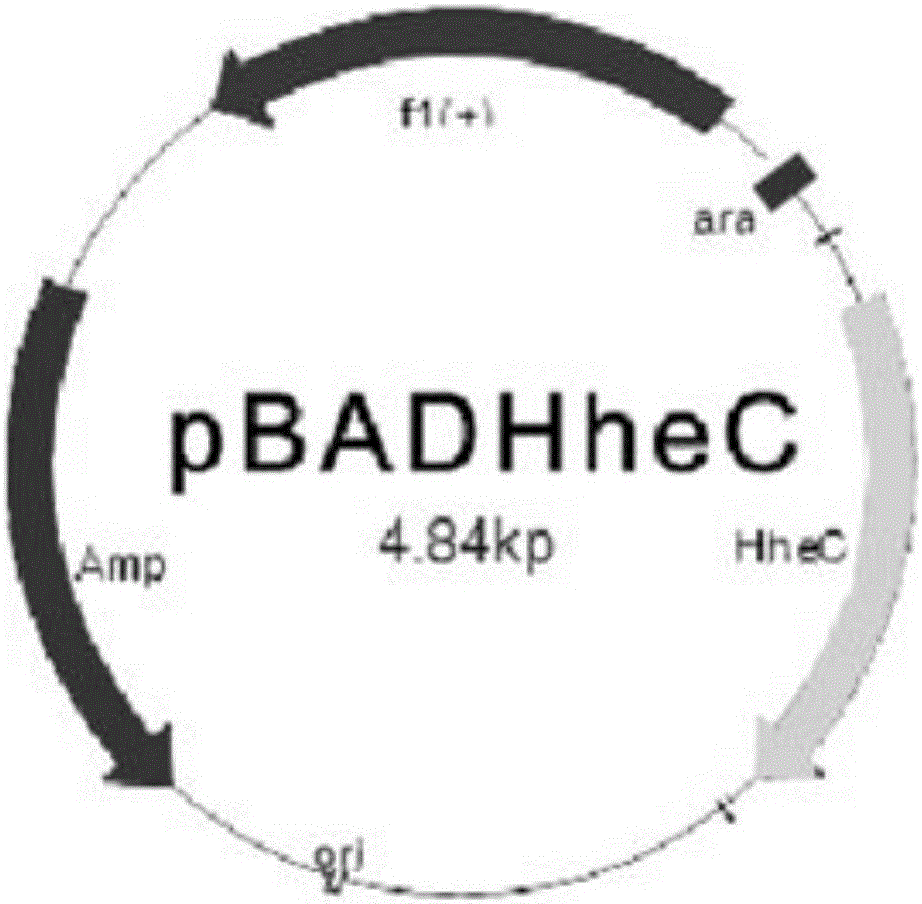
[0042] A1: Transfer the recombinant halohydrin dehalogenase gene into the host cell E.coli MC1061 to obtain the recombinant strain E.coliMC1061, such as figure 1 Shown is a schematic diagram of the recombinant expression of the recombinant halohydrin dehalogenase gene;
[0043] (1) Preparation of competent state:
[0044] Under sterile conditions, the host bacteria E.coli MC1061 was streak-grown on solid LB medium and cultured overnight at 37°C upside down. Pick a single clone from the bacterial plate, inoculate it into fresh LB liquid medium, and culture it on a shaker at a temperature of 37°C and a speed of 180rpm for about 5 hours. 600 =0.3~0.6, take 1ml...
Embodiment 2
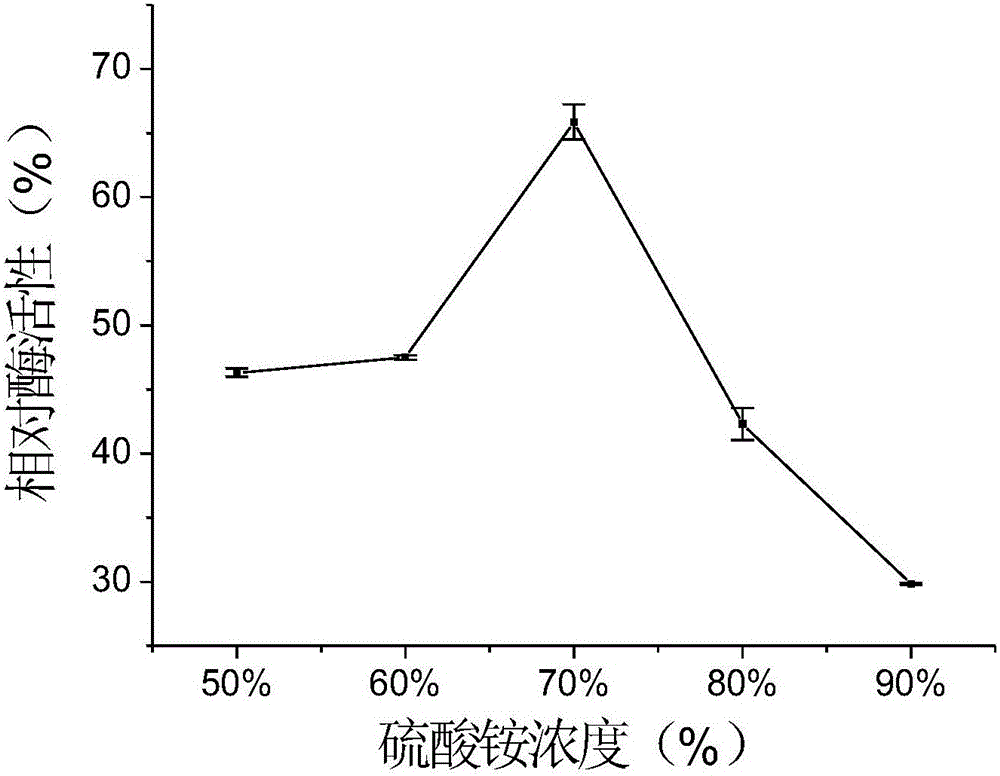
[0056] Effect of different concentrations of precipitant (ammonium sulfate solution) on the enzyme activity of cross-linked halohydrin dehalogenase aggregates:
[0057] In this example, the following reaction system was adopted, and the concentration of ammonium sulfate solution was used as a single variable to complete five parallel experiments of immobilized halohydrin dehalogenases:
[0058] Under the condition of ice bath, take five groups of 1ml of haloalcohol dehalogenation crude enzyme solution with a concentration of 20mg / ml, add 20mg of bovine serum albumin (BSA) powder and stir for 15 minutes, slowly add 5ml to the five groups of mixed solutions respectively Concentration is 50%, 60%, 70%, 80%, 90% ammonium sulfate solution, then stirs for 1 hour, then slowly adds glutaraldehyde dropwise so that the concentration of glutaraldehyde in the solution is 1%, crosslinks for 2 hours, and prepares Cross-linked halohydrin dehalogenase aggregates were obtained.
[0059] In th...
Embodiment 3
[0061] Effects of different immobilization temperatures on the activity of cross-linked halohydrin dehalogenase aggregates
[0062] In this example, the following reaction system was adopted, and three sets of parallel experiments of immobilized halohydrin dehalogenases were completed with the immobilization temperature as a single variable:
[0063] At the three temperatures of 0°C, 15°C and 30°C, take 1ml of halohydrin dehalogenation crude enzyme solution with a concentration of 20mg / ml, add 20mg of bovine serum albumin (BSA) powder and stir for 15 minutes, and then separately Slowly add 5ml of ammonium sulfate solution with a concentration of 70%, and after stirring for 1 hour, slowly add glutaraldehyde dropwise so that the concentration of glutaraldehyde in the solution is 1%. Enzyme aggregates.
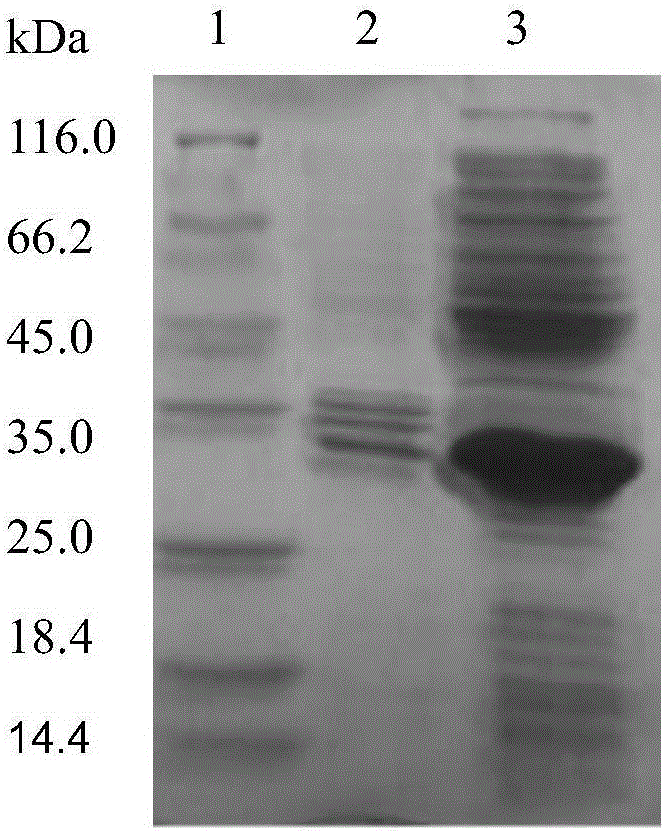
[0064] In this embodiment, the enzyme activity of the three groups of cross-linked halohydrin dehalogenase aggregates prepared above is determined by using the method of measuri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com