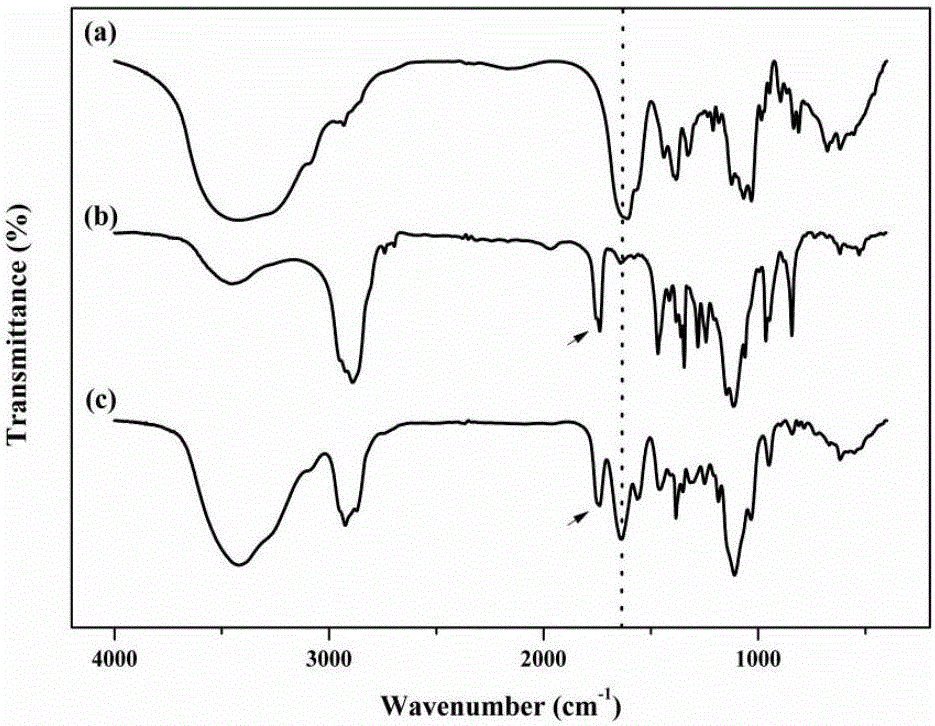
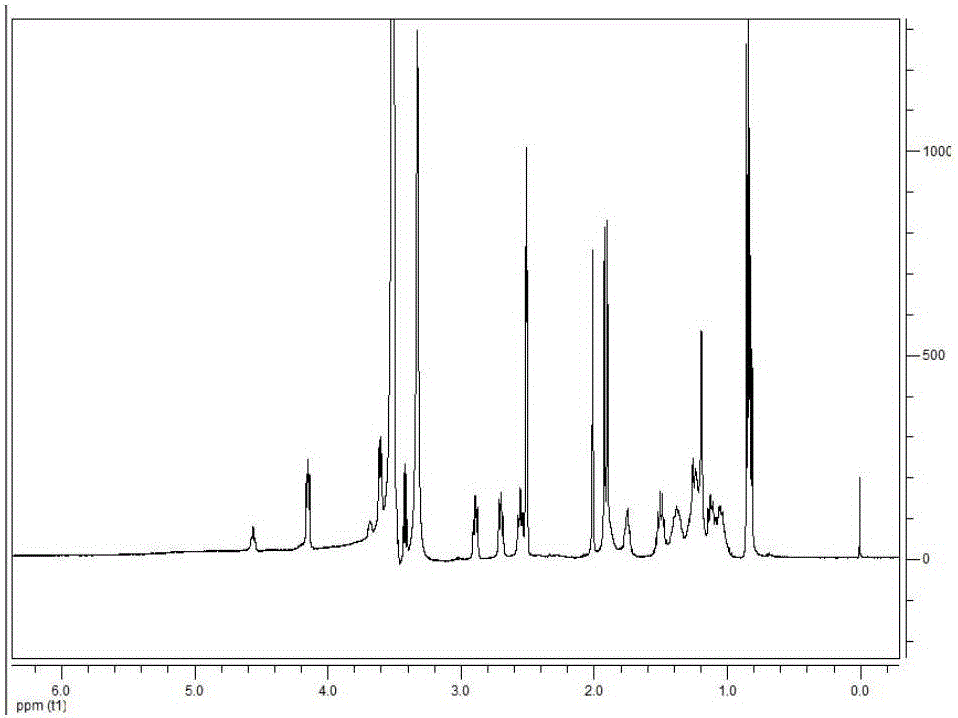
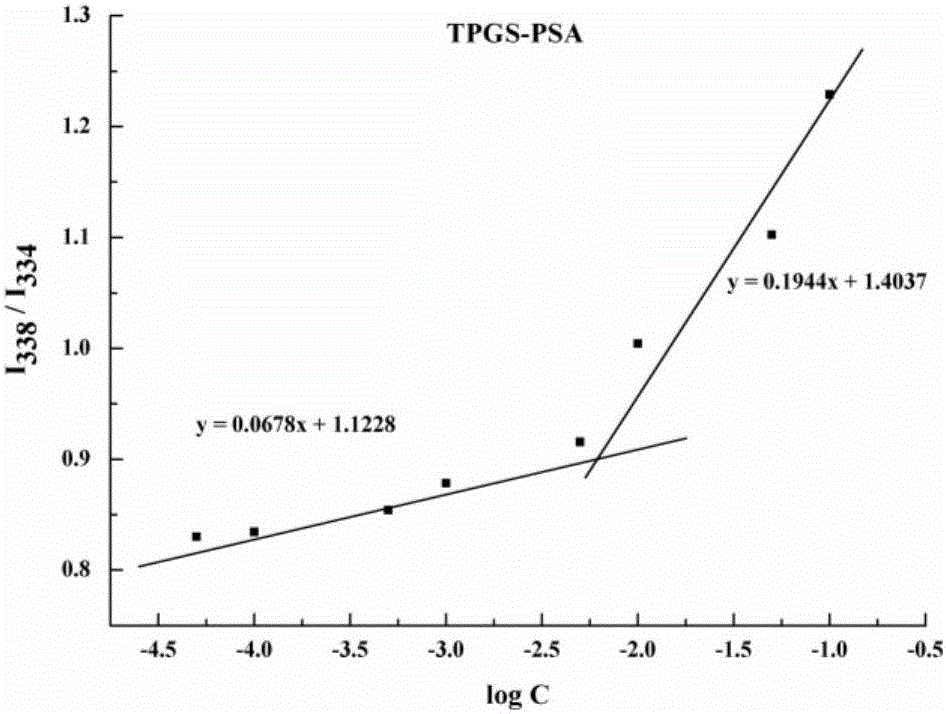
PSA-TPGS (d-alpha-tocopherol polyethylene glycol 1000 succinate) conjugate and application thereof
A technology of conjugates and drugs, applied in PSA‑TPGS conjugates and its application fields, can solve the problems of large differences in TPGS purity, adverse effects of preparations, and uneven purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The synthesis of embodiment 1PSA-TPGS derivative
[0043] 1.1 The preparation method is as follows
[0044] Dissolve PSA (100mg, 0.33mmol SA monomer) in 5mL formamide by heating in a water bath at 60°C. After the system cools down to room temperature, add 80mg N-hydroxysuccinimide (0.68mmol) and 128mg 1-ethyl- (3-Dimethylaminopropyl) carbodiimide hydrochloride (0.68mmol), after stirring for 30min under ice-water bath conditions, TPGS (100mg, 0.066mmol) dissolved in 5mL of formamide was added to the reaction system , filled with nitrogen protection, sealed, and stirred at room temperature for 24 hours. After the reaction was completed, the reaction solution was diluted 2 times with distilled water, and placed in a dialysis bag (molecular weight cut off 10 kDa). The dialysis medium was water system, and the dialysis volume was 3 L. The dialysis medium was changed every 4 hours for 12 hours. After the water dialysis, the dialysis was continued with absolute ethanol, the...
Embodiment 2
[0077] Embodiment 2 film hydration method:
[0078] Accurately weigh 150mg of PSA-TPGS and 5mg of DTX in a round bottom flask, add an appropriate amount of dichloromethane, mix thoroughly by ultrasonication for 10min, remove the organic solvent by rotary evaporation under reduced pressure, and dry in vacuum overnight to remove the residual solvent to obtain dry and transparent drug film. Heat the water bath to 60°C to melt the solid skeleton, add 10 mL of water at the same temperature, and stir for 30 minutes to hydrate. Cool to room temperature and pass through a 0.22 μm filter membrane to obtain a transparent drug-loaded micellar solution. The degree of polymerization of PSA is 100, the molecular weight of PEG in TPGS is 1000, and the encapsulation efficiency is 90%.
Embodiment 3
[0079] Embodiment 3 emulsification solvent volatilization method
[0080] Accurately weigh 150 mg of PSA-TPGS into a vial, add 10 mL of water to dissolve. Dissolve 5mg of DTX in acetone, add it dropwise into the vial quickly under the condition of heating and stirring in a water bath at 40°C, and continue stirring for 10 minutes until the acetone is completely evaporated. Cool to room temperature and pass through a 0.22 μm filter membrane to obtain a transparent drug-loaded micellar solution. The degree of polymerization of PSA is 100, the molecular weight of PEG in TPGS is 1000, and the encapsulation efficiency is 92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com