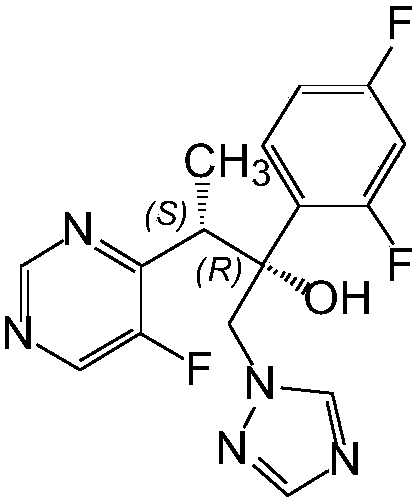
A kind of preparation method of voriconazole and its intermediate
A technology of voriconazole and its intermediates, applied in the field of medicinal chemistry, can solve the problems that the utilization rate of raw and auxiliary materials cannot be greatly improved, the requirements of cost control cannot be met, and the improvement range is limited, so as to achieve easy large-scale industrial production, low cost, and improved utilization rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
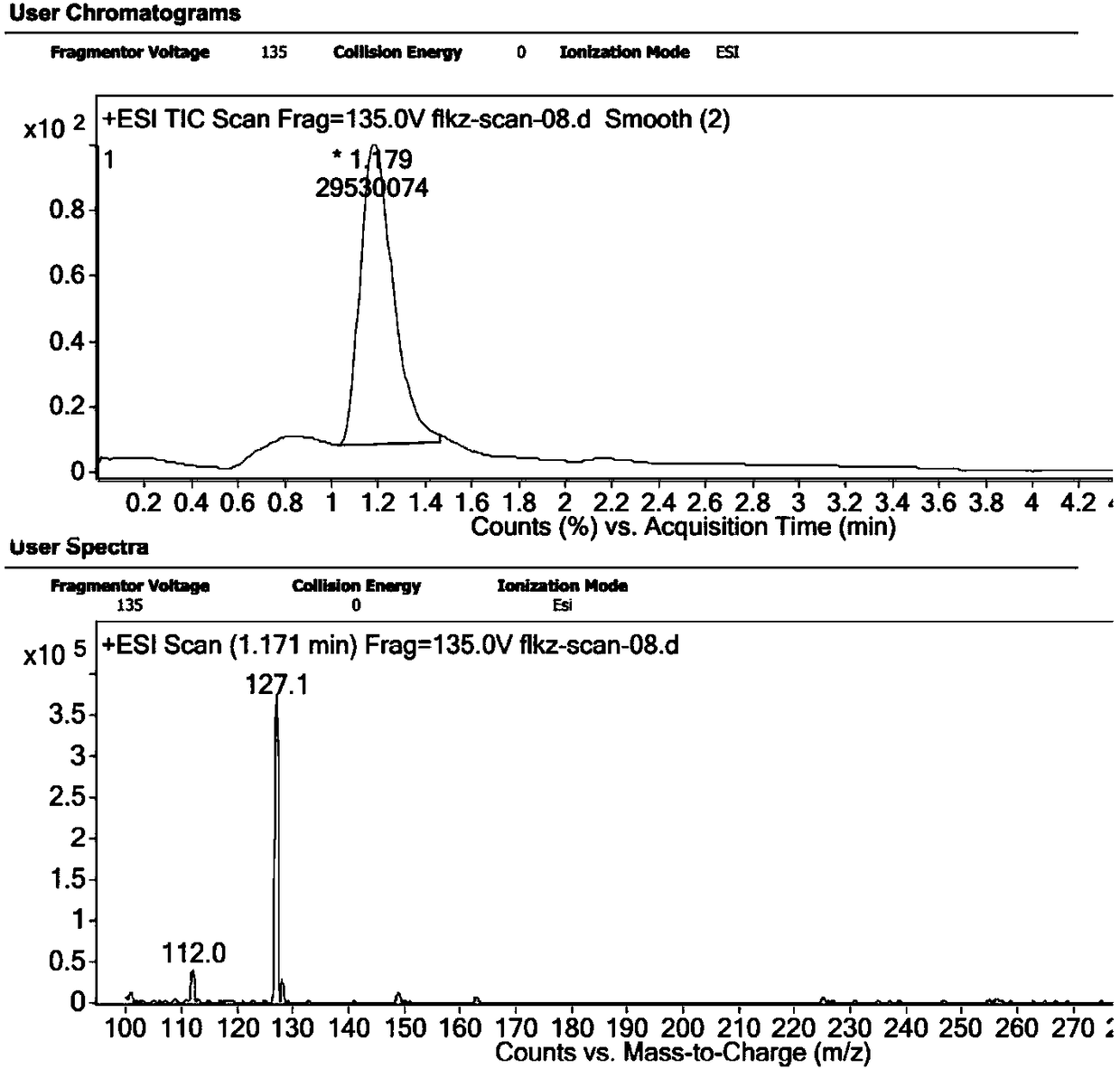
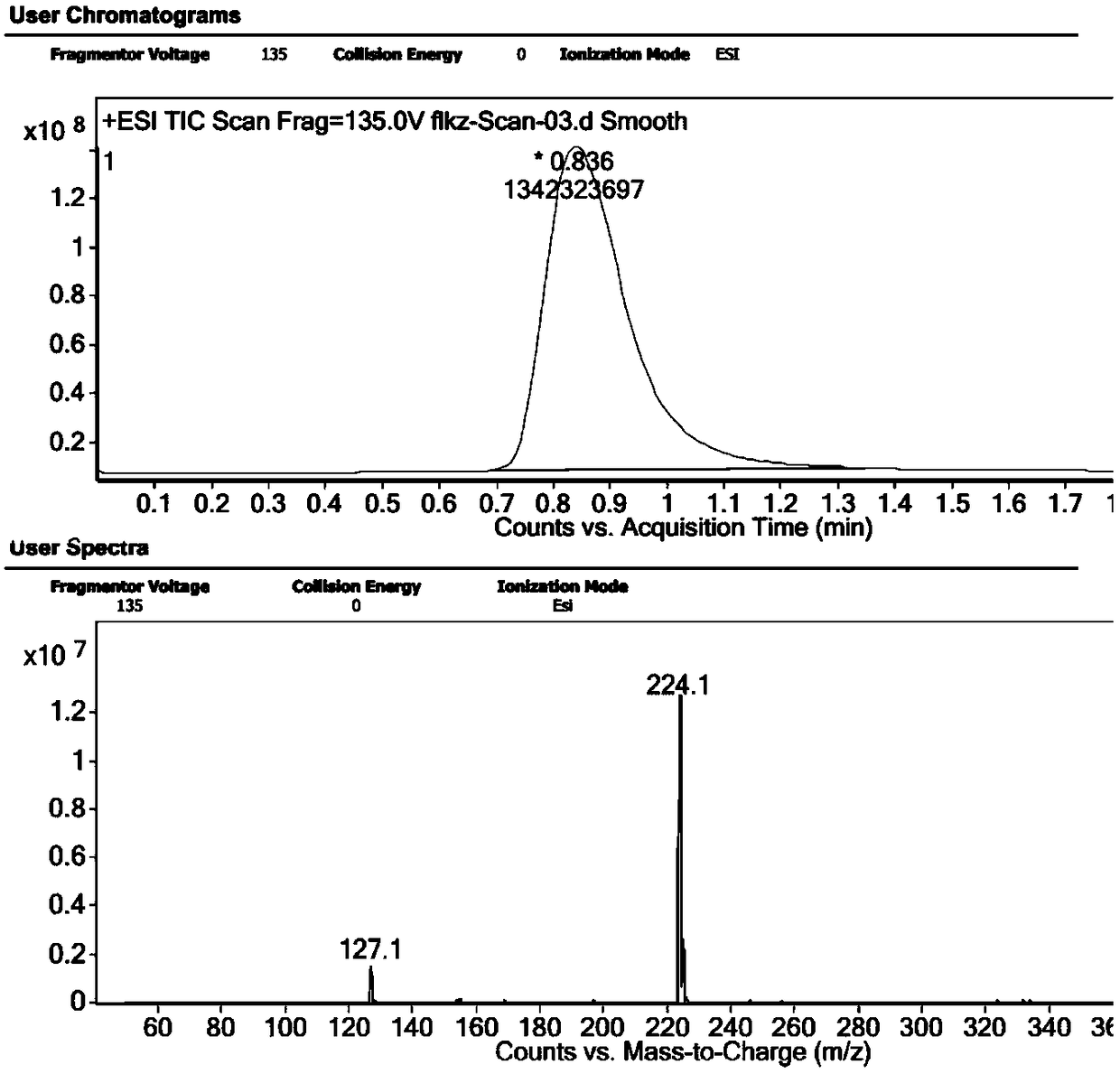
[0035] A preparation method of voriconazole intermediate, which is characterized in that it comprises the following steps: taking voriconazole isomers (2S, 3R) as raw materials, taking 157.5g of raw materials, and reacting in NaOH aqueous solution, the concentration of NaOH is 2mol / L, The reaction temperature is 50°C, and the reaction time is 5 hours. After the reaction, the reaction mixture is cooled, and the mixture of compound II and compound III is first extracted with ethyl acetate, and then concentrated to obtain compound III, and then the collected is concentrated The liquid is subjected to atmospheric distillation to obtain compound II. The LC / MS spectra of compound Ⅱ and compound Ⅲ are attached Figure 5 with 6 .
Embodiment 2
[0037] A preparation method of voriconazole, which is characterized in that it comprises the following steps: taking voriconazole isomers (2S, 3R) as raw materials, taking 157.5 g of raw materials, and reacting in an aqueous NaOH solution, the concentration of NaOH is 1 mol / L, and the reaction temperature The temperature is 50°C and the reaction time is 7 hours. After the reaction, the reaction mixture is cooled and extracted with ethyl acetate to obtain a mixture of compound II and compound III, and then concentrated to obtain compound III, and then the collected concentrate is subjected to normal Pressure distillation to obtain compound II. The compound II was brominated with NBS (refer to the corresponding literature) to obtain 4-(1-bromoethyl)-5-fluoropyrimidine. Then add the reaction reagent, and carry out the Reformatsky reaction and condensation reaction with compound Ⅲ to prepare the racemate of voriconazole, and then add the chiral reagent (L)-camphor-10-sulfonic acid ...
Embodiment 3
[0039] A preparation method of voriconazole intermediate, which is characterized in that it comprises the following steps: taking a mixture of voriconazole isomers (2R, 3R / 2S, 3S / 2S, 3R) as raw materials, taking 174.5 g of raw materials, and reacting in NaOH aqueous solution The concentration of NaOH is 5mol / L, the reaction temperature is 45℃, and the reaction time is 3 hours. After the reaction, the reaction mixture is cooled and extracted with dichloromethane to obtain a mixture of compound II and compound III, and then concentrated to obtain the compound Ⅲ, and then carry out atmospheric distillation on the collected concentrated liquid fraction to obtain compound Ⅱ. The mass spectra of compound Ⅱ and compound Ⅲ are attached Figure 5 with 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com