Method for measuring sitagliptin-related matters by means of separation
A technology related to substances and impurities, applied in the field of drug analysis and detection, can solve the problems of low sensitivity and inaccuracy, and achieve the effect of good separation effect, fast speed and high detection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 system practicability
[0049] Instrument: Agilent1260;
[0050]Chromatographic column: Luna 5μC18(2)100A 250×4.6mm, Guangzhou Philome Scientific Instruments Co., Ltd.
[0051] The mobile phase flow rate is 1.0mL / min;
[0052] The temperature of the chromatographic column is 30°C;
[0053] The injection volume is 10 μL;
[0054] The detection wavelength is 210nm;
[0055] Mobile phase A is 0.5 mol / L potassium dihydrogen phosphate buffer solution, adjusted to Ph=6.2 with 2 mol / L potassium hydroxide; mobile phase B is acetonitrile. The gradient of the mobile phase was set as follows:
[0056] t(min)
A
B
0
82
18
6
82
18
7
69
31
18
69
31
20
30
70
30
30
70
[0057] Reference solution (a): Weigh 25mg of sitagliptin standard substance, accurately weigh it in a 50mL volumetric flask, dilute to the mark with diluent, and mix well. (Sitagliptin concentration: 0.5mg / mL) ...
Embodiment 2
[0072] Example 2 Specificity
[0073] Experimental conditions, liquid chromatography method and solution preparation are as in Example 1.
[0074] Method specificity studies examine peak identification and selectivity. Inject blank solution, impurity localization solution and resolution solution respectively, and record the chromatograms.
[0075] The results are shown in Table 2, and the spectrum is shown in figure 1 ,2,3,4,5.
[0076] Table 2 Specificity
[0077]
[0078]
[0079] The results show that good separation can be achieved between each substance and main component of sitagliptin, and between all related substances.
Embodiment 3
[0080] Embodiment 3 detection limit and quantitative limit
[0081] Experimental conditions, liquid chromatography method and solution preparation are as in Example 1.
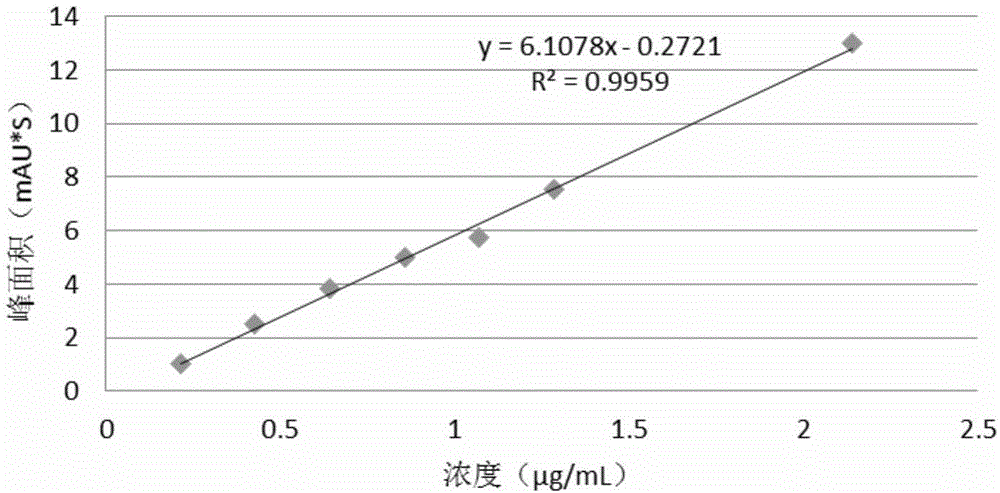
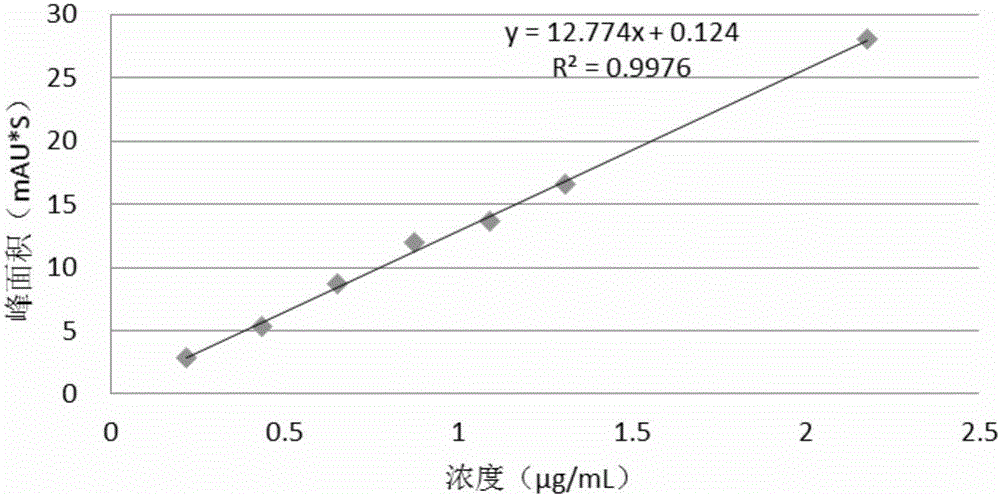
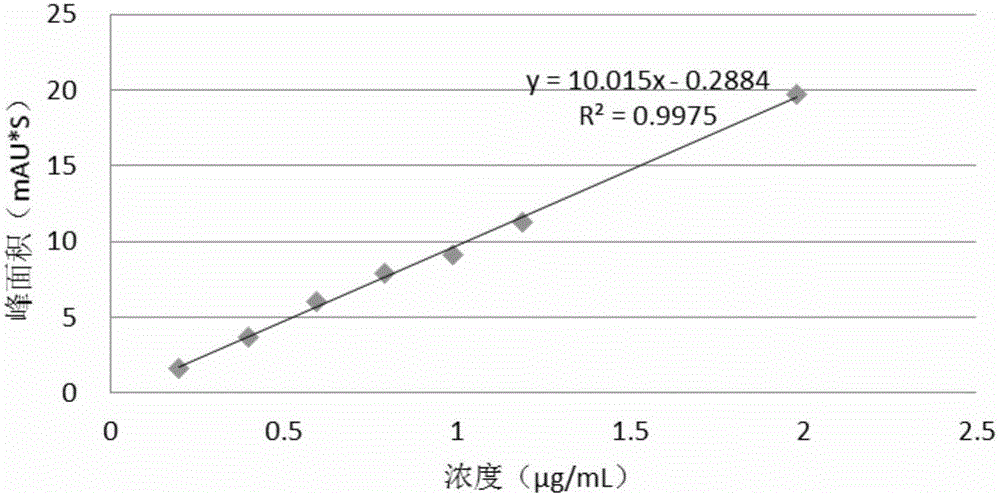
[0082] For known potential impurities, the limit of detection (LOD) and limit of quantitation (LOQ) were determined based on the signal-to-noise ratio method. Dilute the impurity stock solution of known concentration to the sample with the lowest concentration, detect the signal-to-noise ratio S / N-10 to determine the LOQ of the system, and determine the LOD of the system according to S / N-3. For unknown impurities, use sitagliptin samples instead to investigate the detection limit and quantification limit of a single unknown impurity.
[0083] The test results are shown in Tables 3, 4, 5, and 6.
[0084] Table 3 impurity II-2 detection limit and limit of quantitation determination result
[0085]
[0086] Table 4 unknown impurity detection limit and limit of quantitation assay results
[0087]
[0088...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com