Compound capable of effectively inhibiting or killing multi-drug resistant bacteria and preparation method and application of compound
A compound, aldol condensation reaction technology, applied in the field of medicine, can solve problems such as drug-resistant bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Embodiment 1, preparation compound D3
[0206]
[0207] 1. Preparation of intermediate α, β-unsaturated ketone a
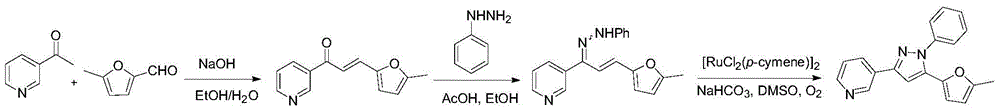
[0208] The specific operation steps are as follows: add 20.0 mL of absolute ethanol to a round bottom flask, cool to 0°C with an ice-water bath, add 1.10 mL (10 mmol) of 3-acetylpyridine and 1.00 mL (10 mmol) of 5-methylfurfural, and dissolve 480 mg Solid sodium hydroxide was dissolved in a mixture of 10.0 mL ethanol and 10.0 mL water, and the sodium hydroxide solution was added dropwise to the substrate solution, and reacted for 2 hours. Add saturated ammonium chloride solution to adjust the reaction solution to neutrality, add 100ml water to dilute, then extract 3 times with dichloromethane (adding 25ml dichloromethane each time), combine the organic phases extracted three times, wash twice with water Dry with anhydrous sodium sulfate, then spin dry the solvent under reduced pressure, and pass the residue through a silica gel column with petroleum eth...
Embodiment 2-15
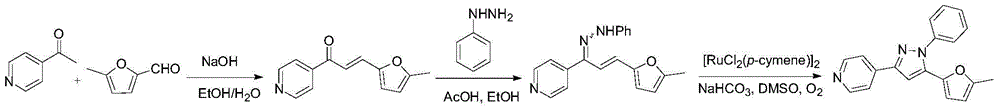
[0220] According to the method basically the same as in Example 1, it is only necessary to replace 3-acetylpyridine or 5-methylfurfural in Step 1 of Example 1 and phenylhydrazine in Step 2 accordingly, and other operations are the same as in Example 1 , to obtain the following compound.
[0221]
[0222] 4-(5-(5-methylfuran-2-yl)-1-phenyl-1H-pyrazol-3-yl)pyridine
[0223] 1 H-NMR (400MHz, CDCl 3 )δ (ppm) 8.628 (d, J = 5.12Hz, 2H), 7.756 (d, J = 5.12Hz, 2H), 7.428-7.464 (m, 5H), 7.006 (s, 1H), 5.906 (d, J =2.52Hz, 1H), 5.782(d, J=3.16Hz, 1H), 2.284(s, 3H);
[0224] 13 C-NMR (100MHz, CDCl 3 )δ (ppm) 152.94, 150.27, 149.34, 142.25, 140.34, 140.11, 136.84, 129.21, 129.00, 126.20, 120.14, 110.22, 107.52, 102.96, 13.55;
[0225] LC-MS (ESI + ): m / z calculated for C 19 h 16 N 3 O(M+H) + :302.13, found 302.22.
[0226]
[0227] 2-(5-(5-methylfuran-2-yl)-1-phenyl-1H-pyrazol-3-yl)pyridine
[0228] 1 H-NMR (400MHz, CDCl 3 )δ (ppm) 8.659 (d, J = 4.74Hz, 1H), 8.046 (d,...
Embodiment 16
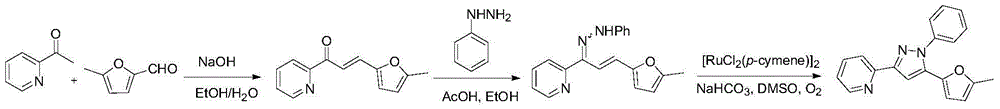
[0288] Example 16, preparation of compound D3-5
[0289]
[0290] Example 16 is similar to 1-15, but the preparation method of the intermediate hydrazone is different.
[0291] In Example 16, unsaturated ketones were prepared by aldol condensation, unsaturated hydrazones were generated by dehydration condensation catalyzed by concentrated sulfuric acid, and pyrazole products were generated by intramolecular carbon-nitrogen bond coupling catalyzed by transition metals.
[0292] Compound data are characterized as follows:
[0293] 1 H-NMR (400MHz, CDCl 3)δ (ppm) 9.108 (s, 1H), 8.617 (d, J = 4.44Hz, 1H), 8.318 (d, J = 8.80Hz, 2H), 8.203 (d, J = 7.92Hz, 1H), 7.689 ( d, J=8.84Hz, 2H), 7.379(dd, J=7.88Hz, J=4.84Hz, 1H), 6.983(s, 1H), 6.256(d, J=3.16Hz, 1H), 6.064(d, J=3.12Hz, 1H), 2.300(s, 3H);
[0294] 13 C-NMR (100MHz, CDCl 3 )δ (ppm) 153.84, 150.40, 149.61, 147.39, 146.60, 145.03, 141.16, 136.21, 133.05, 128.19, 125.04, 124.45, 123.66, 111.72, 107.74, 105.45, 13.58;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com